Immunotherapy targeting a leader sequence cathepsin G-derived peptide
- PMID: 39939820
- PMCID: PMC11976275
- DOI: 10.1038/s41375-025-02520-x
Immunotherapy targeting a leader sequence cathepsin G-derived peptide
Abstract
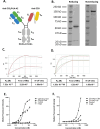
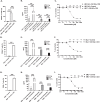
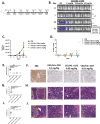
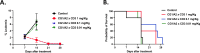
Myeloid azurophil granules provide a rich source of intracellular leukemia antigens. Cathepsin G (CG) is a serine protease that has higher expression in acute myeloid leukemia (AML) blasts in comparison to normal myeloid progenitors. Based on the unique biology of HLA-A*0201 (HLA-A2), in which presentation of leader sequence (LS)-derived peptides is favored, we focused on the LS-CG-derived peptide CG1 (FLLPTGAEA). We previously detected CG1/HLA-A2 complexes on the surface of primary HLA-A2+ AML blasts and cell lines, and immunity targeting CG1/HLA-A2 in leukemia patients. T cell receptor (TCR)-mimic (m) antibodies are immunotherapeutic antibodies that target peptide-HLA (pHLA) complexes. Here we report on the engineering, preclinical efficacy, and safety evaluation of a novel CG1/HLA-A2-targeting, T cell-engager, bispecific antibody (CG1/A2xCD3). CG1/A2xCD3 showed high binding affinity to CG1/HLA-A2 monomers, CD3-Fc fusion protein, and to AML and T cells, with potent killing of HLA-A2+ primary AML and cell lines in vitro and in vivo. This correlated with both tumor- and CG1/A2xCD3-dependent T cell activation and cytokine secretion. Lastly, CG1/A2xCD3 had no activity against normal bone marrow. Together, these results support the targeting of LS-derived peptides and the continued clinical development of CG1/A2xCD3 in the setting of AML.
© 2025. The Author(s).
Conflict of interest statement
Ethics approval and consent to participate: All methods were performed in accordance with the University of Texas MD Anderson Cancer Center guidelines and regulations for laboratory research. Research approval was obtained through the MD Anderson Cancer Center IRB-approved tissue collection protocol (Protocol# LAB99-062) and IACUC animal protocol (Protocol# 00001336-RN02). Informed consent was obtained from all study participants prior to tissue collection. This study did not contain any direct research on human subjects; only archived human tissue. No identifiable images from human research participants were included in this manuscript. Competing interests: The authors declare no competing interests.
Figures

References
-
- Holmberg-Thyden S, Dufva IH, Gang AO, Breinholt MF, Schejbel L, Andersen MK, et al. Epigenetic therapy in combination with a multi-epitope cancer vaccine targeting shared tumor antigens for high-risk myelodysplastic syndrome—a phase I clinical trial. Cancer Immunol Immunother. 2022;71:433–44. - PMC - PubMed
-
- Molldrem JJ, Clave E, Jiang YZ, Mavroudis D, Raptis A, Hensel N, et al. Cytotoxic T lymphocytes specific for a nonpolymorphic proteinase 3 peptide preferentially inhibit chronic myeloid leukemia colony-forming units. Blood. 1997;90:2529–34. - PubMed
-
- Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med. 2000;6:1018–23. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

