Copper-enriched automotive brake wear particles perturb human alveolar cellular homeostasis
- PMID: 39940013
- PMCID: PMC11823208
- DOI: 10.1186/s12989-024-00617-2
Copper-enriched automotive brake wear particles perturb human alveolar cellular homeostasis
Abstract
Background: Airborne fine particulate matter with diameter < 2.5 μm (PM2.5), can reach the alveolar regions of the lungs, and is associated with over 4 million premature deaths per year worldwide. However, the source-specific consequences of PM2.5 exposure remain poorly understood. A major, but unregulated source is car brake wear, which exhaust emission reduction measures have not diminished.
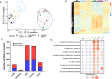
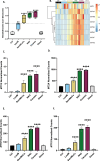
Methods: We used an interdisciplinary approach to investigate the consequences of brake-wear PM2.5 exposure upon lung alveolar cellular homeostasis using diesel exhaust PM as a comparator. This involved RNA-Seq to analyse global transcriptomic changes, metabolic analyses to investigate glycolytic reprogramming, mass spectrometry to determine PM composition, and reporter assays to provide mechanistic insight into differential effects.
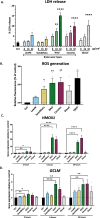
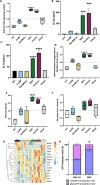
Results: We identified brake-wear PM from copper-enriched non-asbestos organic, and ceramic brake pads as inducing the greatest oxidative stress, inflammation, and pseudohypoxic HIF activation (a pathway implicated in diseases associated with air pollution exposure, including cancer, and pulmonary fibrosis), as well as perturbation of metabolism, and metal homeostasis compared with brake wear PM from low- or semi-metallic pads, and also, importantly, diesel exhaust PM. Compositional and metal chelator analyses identified that differential effects were driven by copper.
Conclusions: We demonstrate here that brake-wear PM may perturb cellular homeostasis more than diesel exhaust PM. Our findings demonstrate the potential differences in effects, not only for non-exhaust vs exhaust PM, but also amongst different sources of non-exhaust PM. This has implications for our understanding of the potential health effects of road vehicle-associated PM. More broadly, our findings illustrate the importance of PM composition on potential health effects, highlighting the need for targeted legislation to protect public health.
Keywords: Alveolar; Brake-wear PM; Copper; Diesel PM; Epithelium; Hypoxia-inducible factor (HIF); Metallothionein; Non-exhaust emissions; Non-tailpipe emissions; Pseudohypoxic signalling.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Primary ATII cells were extracted from macroscopically normal human lung tissue deemed surplus to clinical diagnostic requirements, obtained following video assistant thoracoscopic surgery (VATS). Ethical approval was gained from the Southampton and South West Hampshire and the Mid and South Buckinghamshire Local Research Ethics Committees, and all subjects gave written informed consent. Consent for publication: Not applicable. Competing interests: JD has acted as a consultant for AstraZeneca, Jubilant, Theras, Roche and Vividion and has funded research agreements with Bristol Myers Squibb, Revolution Medicines and AstraZeneca. DED. is co-founder of, shareholder in, and consultant to Synairgen Research Ltd. DED and MGJ acknowledge grants from Boehringer Ingelheim. ML is a member of the UK expert Committee on the Medical Effects of Air Pollutants (COMEAP). All other authors declare they have no competing interests.
Figures



References
-
- WHO. WHO global air quality guidelines. Particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide.: WHO; 2021. Report No.: 978-92-4-003422-8. - PubMed
-
- Miller MR, Newby DE. Air pollution and cardiovascular disease: car sick. Cardiovasc Res. 2020;116(2):279–94. - PubMed
-
- Kim KH, Kabir E, Kabir S. A review on the human health impact of airborne particulate matter. Environ Int. 2015;74:136–43. - PubMed
-
- Rana S, Saxena MR, Maurya RK. A review on morphology, nanostructure, chemical composition, and number concentration of diesel particulate emissions. Environ Sci Pollut Res Int. 2022;29(11):15432–89. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

