Food oral immunotherapy
- PMID: 39940042
- PMCID: PMC11823072
- DOI: 10.1186/s13223-025-00948-5
Food oral immunotherapy
Abstract
Food oral immunotherapy (OIT) is an option for the treatment of immunoglobin E (IgE)-mediated food allergy that involves administering gradually increasing doses of an allergenic food over time (under medical supervision) with the goal of desensitizing an individual to the food allergen. Current Canadian clinical practice guidelines for OIT recommend this form of therapy as an option in patients with food allergy. The intervention should be prioritized in the infant and toddler population, in which it is particularly well tolerated and can lead to sustained unresponsiveness (also sometimes referred to as remission). In this article, we provide an overview of OIT and discuss the role non-allergist clinicians can play in caring for patients undergoing OIT.
© 2025. Crown.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethics approval and consent to participate are not applicable to this review article. Consent for publication: Not applicable. Competing interests: Dr. Mary McHenry has received consulting fees and honoraria from Sanofi Genzyme and Medexus, and is on the Board of Directors for CAAIF. Dr. Philippe Bégin has received consulting fees and honoraria from ALK, Astra Zeneca, Sanofi, Bausch Health, Pfizer. He is also a clinical trial investigator with DBV Technologies (Viaskin Patch), Regeneron, Novartis, ALK, and Sanofi. He is on the Board of Directors of the CSACI, AAIQ and CAAIF and medical advisor to Food Allergy Canada. Dr. Edmond Chan has received research support from DBV Technologies; has been a member of advisory boards for Pfizer, Miravo, Medexus, Leo Pharma, Kaleo, DBV, AllerGenis, Sanofi Genzyme, Bausch Health, Avir Pharma, AstraZeneca, ALK, Alladapt; was co-lead of the CSACI oral immunotherapy guidelines; is on the Executive of the CSACI (Canadian Society of Allergy and Clinical Immunology); is on the Executive of the CPS (Canadian Paediatric Society) Allergy Section; and is a member of the healthcare advisory board for Food Allergy Canada. Dr. Meriem Latrous has received consulting fees and honoraria from Sanofi, ALK, Pfizer, Miravo, and Bausch Health. Dr. Harold Kim has received honoraria and/or consulting fees from ALK, AstraZeneca, Bausch Health, CSL Behring, GSK, Miravo, Novartis, Medexus, Pfizer, Sanofi, Shire, Takeda.
Figures



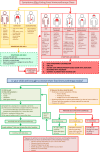
References
-
- Nurmatov U, Dhami S, Arasi S, Pajno GB, Fernandez-Rivas M, Muraro A, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72(8):1133–47. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

