Oral Probiotics to Prevent Recurrent Vulvovaginal Infections During Pregnancy-Multicenter Double-Blind, Randomized, Placebo-Controlled Trial
- PMID: 39940318
- PMCID: PMC11820037
- DOI: 10.3390/nu17030460
Oral Probiotics to Prevent Recurrent Vulvovaginal Infections During Pregnancy-Multicenter Double-Blind, Randomized, Placebo-Controlled Trial
Abstract
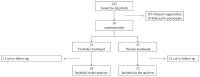
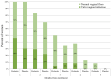
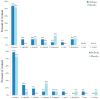
Objective: During pregnancy, vulvovaginal infections (VVIs), including abnormal vaginal flora (AVF), bacterial vaginosis (BV), and vulvovaginal candidiasis (VVC), are associated with serious complications and discomfort. We aimed to elucidate the effectiveness of oral probiotics in secondary prevention of VVIs in pregnant women. Study design: A multicenter prospective randomized, double-blind, placebo-controlled trial was conducted at three medical centers between 2016 and 2021. Women who complained of vaginal symptoms with positive smear for AVF/BV and/or candida were treated with antibiotics or an antimycotic agent, respectively. After confirmation of VVI eradiation by repeated vaginal smear, the women were divided into a research group, receiving two capsules/day of oral probiotic formula containing Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus (L.) acidophilus, L. paracasei, L. rhamnosus and Streptococcus thermophilus (>6 × 109 CFU/capsule), and a control group, receiving a placebo (two capsules/day) until delivery. At least once a month or following complaints, a vaginal smear was taken to assess vaginal microbiota. If VVIs were found, they were treated with antibiotics/antimycotics, and eradication was assessed by a repeated vaginal smear. Lactobacilli vaginal colonization, including the specific strains from the probiotic capsules, were detected using the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI TOF-MS). The primary outcome was the rate of women who developed VVI during the study period until delivery. Results: Twenty-three and twenty-four women were analyzed in the probiotic and placebo cohorts, respectively. There was no difference in the rate of any VVI between the probiotic and placebo cohorts (16 (67%) versus 11 (48%), respectively; p = 0.19), time until first infection or pregnancy outcomes. The lactobacilli strains that colonized the vagina were similar at baseline and following probiotic or placebo administration. No woman was detected with vaginal colonization of the strains from the capsule, although the probiotics were taken for about 4 months. Conclusions: The oral probiotic product tested in this study did not reduce the recurrence rate of VVIs in pregnant women following eradication.
Keywords: abnormal vaginal flora; bacterial vaginosis; pregnancy; probiotics; secondary prevention; vulvovaginal candidiasis; vulvovaginal infections.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures

References
-
- Carey J.C., Klebanoff M.A., Hauth J.C., Hillier S.L., Thom E.A., Ernest J.M., Heine R.P., Nugent R.P., Fischer M.L., Leveno K.J., et al. Metronidazole to Prevent Preterm Delivery in Pregnant Women with Asymptomatic Bacterial Vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N. Engl. J. Med. 2000;342:534–540. doi: 10.1056/NEJM200002243420802. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

