The Ferroxidase-Permease System for Transport of Iron Across Membranes: From Yeast to Humans
- PMID: 39940646
- PMCID: PMC11817551
- DOI: 10.3390/ijms26030875
The Ferroxidase-Permease System for Transport of Iron Across Membranes: From Yeast to Humans
Abstract
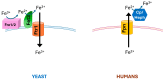
Transport of iron across the cell membrane is a tightly controlled process carried out by specific proteins in all living cells. In yeast and in mammals, a system formed by an enzyme with ferroxidase activity coupled to a membrane transporter supports iron uptake or iron efflux, respectively. Ferroxidase belongs to the family of blue multicopper oxidases, enzymes able to couple the one-electron oxidation of substrate(s) to full reduction of molecular oxygen to water. On the other hand, the permeases are widely different and are specific to Fe3+ and Fe2+ in yeast and multicellular organisms, respectively. This review will describe the yeast and human ferroxidase-permease systems, highlighting similarities and differences in structure, function and regulation of the respective protein components.
Keywords: Fet3; Ftr1; ceruloplasmin; copper; ferroportin; ferroxidase; hephaestin; iron; multicopper oxidase; yeast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

