Anti-Inflammatory Effects of Algae-Derived Biomolecules in Gut Health: A Review
- PMID: 39940655
- PMCID: PMC11817955
- DOI: 10.3390/ijms26030885
Anti-Inflammatory Effects of Algae-Derived Biomolecules in Gut Health: A Review
Abstract
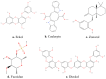
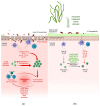
Under physiological conditions, the inflammatory response acts as a biological defense against tissue damage or infection, and is rapidly resolved once the infection is cleared. However, chronic inflammatory diseases, including inflammatory bowel disease (IBD), have become increasingly widespread in the last decades, placing a burden on the quality of life of affected people and on healthcare systems worldwide. Available drug therapies are often ineffective due to the chronic nature of these diseases, and prolonged administration of drugs can result in severe side effects for the patient or a lack of efficacy. In addition, there is the growing problem of bacterial resistance to synthetic antibiotics. Together, these factors have led to a strong research focus on the discovery of natural products capable of treating IBD. Recently, there has been a growing interest in compounds derived from marine sources, mainly algae, due to their bioactive secondary metabolites with anti-inflammatory properties well known in the literature. Based on this evidence, this review aimed to evaluate the anti-inflammatory potential of algae-derived biomolecules in IBD. In particular, interesting species from green algae (e.g., Chlorella vulgaris and Ulva pertusa), brown algae (e.g., Macrocystis pyrifera and Ecklonia cava) and red algae (e.g., Porphyra tenera and Grateloupia turuturu) are included in this review due to their proven anti-inflammatory properties. For this purpose, an extensive literature search was conducted using several databases. The results suggest that both macroalgae and microalgae have remarkable potential for IBD therapy due to the anti-inflammatory and antioxidant activities of their bioactive compounds. However, while the preclinical evidence is encouraging, further and long-term clinical studies are needed to better understand their mechanisms of action in order to determine the true efficacy of marine algae in the treatment of IBD.
Keywords: IBD; algae; biomolecules; gut inflammation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Molodecky N.A., Soon I.S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G., Benchimol E.I., Panaccione R., Ghosh S., Barkema H.W., et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

