Assessment of Torquetenominivirus (TTMV) and Torquetenomidivirus (TTMDV) as Complementary Biomarkers to Torquetenovirus (TTV)
- PMID: 39940791
- PMCID: PMC11817373
- DOI: 10.3390/ijms26031022
Assessment of Torquetenominivirus (TTMV) and Torquetenomidivirus (TTMDV) as Complementary Biomarkers to Torquetenovirus (TTV)
Abstract
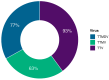
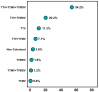
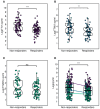
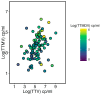
Recent studies have identified Torquetenovirus (TTV) as a promising biomarker of immune competence, particularly in assessing the vaccine response of solid organ transplant (SOT) recipients. However, given the individual variability of viral load, it is not yet possible to define "normal levels". Nevertheless, TTV is just one component of the broader Anelloviridae family, which also includes Torquetenominivirus (TTMV) and Torquetenomidivirus (TTMDV). This study explores whether the viremia of TTMV and TTMDV offers a stronger predictive marker for vaccine efficacy in SOT recipients. A cohort of 168 SOT patients (142 kidney and 26 lung transplant recipients) who received the BNT162B2 mRNA vaccine was examined, with viral loads quantified through virus-specific real-time PCR. While TTV remains a potentially useful biomarker for evaluating immune response, the combined analysis of all anelloviruses viremia provides deeper insights, particularly in cases where TTV is undetectable. Notably, only TTMV exhibited a pattern similar to TTV, suggesting its potential as an alternative biomarker when TTV is absent from the patient's virome.
Keywords: COVID-19; SOT; Torquetenomidivirus; Torquetenominivirus; Torquetenovirus; anellovirus; biomarker.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Timsit J.-F., Sonneville R., Kalil A.C., Bassetti M., Ferrer R., Jaber S., Lanternier F., Luyt C.-E., Machado F., Mikulska M., et al. Diagnostic and Therapeutic Approach to Infectious Diseases in Solid Organ Transplant Recipients. Intensive Care Med. 2019;45:573–591. doi: 10.1007/s00134-019-05597-y. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2022ZRNTHW/Italian Ministry of University and Research
- PE00000007/EU funding within the MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (INF-ACT)
- 896932/Italian Ministry of Health "Ricerca Corrente - Linea 1 - INMI L. Spallanzani I.R.C.C.S." funding and by the European Union's Horizon 2020 research and innovation programme (TTVguideTX project)
LinkOut - more resources
Full Text Sources

