Loss of Tyrosine Phosphatase Mu Promotes Scoliosis Progression Through Osteopontin-α5β1 Integrin Signaling and PIPK1γ90 Activity
- PMID: 39940812
- PMCID: PMC11816665
- DOI: 10.3390/ijms26031042
Loss of Tyrosine Phosphatase Mu Promotes Scoliosis Progression Through Osteopontin-α5β1 Integrin Signaling and PIPK1γ90 Activity
Abstract
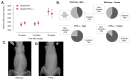
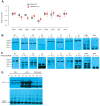
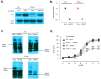
Adolescent idiopathic scoliosis (AIS) is characterized by a curvature of the spine affecting approximately 4% of the pediatric population, and the mechanisms driving its progression remain poorly understood. Whole-exome sequencing of a French-Canadian AIS cohort with severe scoliosis identified rare variants in the PTPRM gene, which encodes Protein Tyrosine Phosphatase μ (PTPµ). However, these rare variants alone did not account for the pronounced reduction in PTPµ at both mRNA and protein levels in severe AIS cases. This led us to investigate epigenetic regulators and the identification of five microRNAs (miR-103a-3p, miR-107, miR-148a-3p, miR-148b-3p, and miR-152-3p) that target PTPRM mRNA. These microRNAs were significantly elevated in plasma from severe AIS patients, and miR-148b-3p was also upregulated in AIS osteoblasts. Phenotypic analysis of bipedal Ptrprm knockout (PTPµ -/-) mice showed increased prevalence and severity of scoliosis, while quadrupedal PTPµ -/- mice did not develop scoliosis, underscoring PTPµ's role as a disease-modifying factor. Mechanistically, PTPµ deficiency was found to disrupt Gi-coupled receptor signaling in osteoblasts by enhancing the interaction between osteopontin (OPN) and α5β1 integrin, along with increased tyrosine phosphorylation of phosphatidylinositol-4-phosphate 5-kinase type I (PIPKIγ90). These findings provide novel insights into the molecular mechanisms underlying spinal deformity progression in AIS, linking PTPµ depletion to aberrant OPN-α5β1 integrin signaling and highlighting potential therapeutic targets to stop, mitigate, or prevent scoliosis.
Keywords: Gi-coupled receptor signaling; OPN-α5β1 integrin; PIPK1γ90; PTPRM variants; PTPµ; PTPµ-null mice; adolescent idiopathic scoliosis; microRNAs; osteoblast; spinal deformity.
Conflict of interest statement
This work led to a patent application (pending) owned by CHU Sainte-Justine. The authors have declared that no other potential conflicts of interest exist.
Figures




References
-
- Dickson R.A. The etiology and pathogenesis of idiopathic scoliosis. Acta Orthop. Belg. 1992;58((Suppl. 1)):21–25. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

