Adult Leigh Syndrome Associated with the m.15635T>C Mitochondrial DNA Variant Affecting the Cytochrome b (MT-CYB) Gene
- PMID: 39940884
- PMCID: PMC11817157
- DOI: 10.3390/ijms26031116
Adult Leigh Syndrome Associated with the m.15635T>C Mitochondrial DNA Variant Affecting the Cytochrome b (MT-CYB) Gene
Abstract
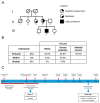
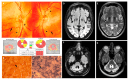
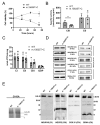
We report on a sporadic patient suffering Leigh syndrome characterized by bilateral lesions in the lenticular nuclei and spastic dystonia, intellectual disability, sensorineural deafness, hypertrophic cardiomyopathy, exercise intolerance, and retinitis pigmentosa. Complete sequencing of mitochondrial DNA revealed the heteroplasmic nucleotide change m.15635T>C affecting a highly conserved amino acid position (p.Ser297Pro) in the cytochrome b (MT-CYB) gene on a haplogroup K1c1a background, which includes a set of four non-synonymous polymorphisms also present in the same gene. Biochemical studies documented respiratory chain impairment due to complex III defect. This variant fulfils the criteria for being pathogenic and was previously reported in a sporadic case of fatal neonatal polyvisceral failure.
Keywords: Leigh syndrome; MT-CYB; Respiratory complex III; mtDNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

