A Non-Pharmacological Paradigm Captures the Complexity in the Mechanism of Action of Poliprotect Against Gastroesophageal Reflux Disease and Dyspepsia
- PMID: 39940951
- PMCID: PMC11818618
- DOI: 10.3390/ijms26031181
A Non-Pharmacological Paradigm Captures the Complexity in the Mechanism of Action of Poliprotect Against Gastroesophageal Reflux Disease and Dyspepsia
Abstract
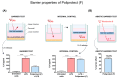
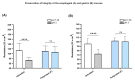
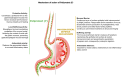
When the protective mechanisms of the gastroesophageal mucosa are overwhelmed by injurious factors, the structural and functional mucosal integrity is compromised, resulting in a wide spectrum of disorders. Poliprotect has recently been shown to be non-inferior to standard-dose omeprazole for the treatment of endoscopy-negative patients with heartburn and/or epigastric pain or burning. Here, we provide preclinical data describing the mechanism of action of the Poliprotect formulation, a 100% natural, biodegradable, and environmental friendly medical device according to EU 2017/745 and containing UVCB (unknown or variable composition, complex-reaction products, or biological materials) substances of botanical and mineral origin, according to the REACH and European Chemical Agency definitions. Different in vitro assays demonstrated the capability of Poliprotect to adhere to mucus-secreting gastric cells and concomitantly deliver a local barrier with buffering and antioxidant activity. In studies conducted in accordance with systems biology principles, we evaluated the effects of this barrier on human gastric cells exposed to acidic stress. Biological functions identified via Ingenuity Pathway Analysis highlighted the product's ability to create a microenvironment that supports the mucosal structural and functional integrity, promotes healing, and restores a balanced mucosal inflammatory status. Additionally, transepithelial electrical resistance and an Ussing chamber showed the product's capability of preserving the integrity of the gastric and esophageal epithelial barriers when exposed to an acid solution. Two in vivo models of erosive gastropathy further highlighted its topical protection against ethanol- and drug-induced mucosal injury. Overall, our findings sustain the feasibility of a paradigm shift in therapeutics R&D by depicting a very innovative and desirable mode of interaction with the human body based on the emerging biophysical, rather than the pharmacological properties of these therapeutic agents.
Keywords: NeoBianacid; Poliprotect; biophysical properties; dyspepsia; emerging properties; gastroesophageal reflux disease; medical device; non-pharmacological mechanism; novel R&D paradigm.
Conflict of interest statement
S.C., C.B., S.G., I.F.M., S.U., A.G., E.P., C.S. and J.L. are employed by Bios’-Therapy, Physiological Systems For Health S.p.A. C.R., L.M. and M.P. received support from the study of the formulation described here. A.M. and A.C. are employed in the research/medical division of Aboca S.p.A. Società Agricola, the manufacturer of Poliprotect(F). S.B.d.V. has received honoraria for speaking at symposia. J.R. is a speaker bureau member for Aboca S.pA. Società Agricola. P.M. is a consultant for Aboca S.p.A. Società Agricola and a speaker bureau member for Alfasigma, Bayer, Biocodex, Menarini, and Phathom.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

