Association Between Active DNA Demethylation and Liver Fibrosis in Individuals with Metabolic-Associated Steatotic Liver Disease (MASLD)
- PMID: 39941038
- PMCID: PMC11818491
- DOI: 10.3390/ijms26031271
Association Between Active DNA Demethylation and Liver Fibrosis in Individuals with Metabolic-Associated Steatotic Liver Disease (MASLD)
Abstract
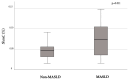
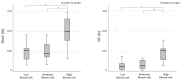
Metabolic-associated steatotic liver disease (MASLD) represents the most common chronic hepatopathy worldwide and an independent risk factor for cardiovascular disease and mortality, particularly when liver fibrosis occurs. Epigenetic alterations, such as DNA methylation, may influence MASLD susceptibility and progression; yet mechanisms underlying this process are limited. This study aimed to investigate whether active DNA demethylation in peripheral blood mononuclear cells (PBMCs) from individuals with MASLD, alongside the methylation and mRNA levels of inflammation- and fibrosis-related candidate genes, is associated with liver fibrosis. For this study, global demethylation intermediates (5-hydroxymethylcytosine [5hmC], 5-formylcytosine [5fC]) were quantified in PBMCs from 89 individuals with/without MASLD using ELISA. Site-specific DNA methylation of SOCS3, SREBF1, and TXNIP was analyzed by mass spectrometry-based bisulfite sequencing; mRNA expression was assessed via RT-PCR. Individuals with MASLD and moderate-to-high fibrosis risk (estimated by the fibrosis non-alcoholic steatohepatitis (NASH) index, FNI) progressively exhibited greater global 5hmC and 5fC levels. Higher FNI was associated with reduced methylation of the SOCS3 gene and increased mRNA expression of the SOCS3, TXNIP, IL-6, and MCP-1 genes. In conclusion, elevated fibrosis risk in MASLD is associated with active global DNA demethylation, as well as differential methylation and expression patterns of genes, which are key regulators of inflammation and fibrosis. These epigenetic alterations in PBMCs may mirror DNA methylation changes in the liver, which may potentially contribute to liver fibrogenesis and represent novel biomarkers for MASLD progression toward fibrosis.
Keywords: DNA demethylation; DNA methylation; FNI; NASH; epigenetics; fibrosis NASH index; liver fibrosis; metabolic-associated fatty liver disease; non-alcoholic fatty liver disease.
Conflict of interest statement
The authors declare no conflicts of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Younossi Z.M., Golabi P., de Avila L., Paik J.M., Srishord M., Fukui N., Qiu Y., Burns L., Afendy A., Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

