Exploiting Silica-Binding and Silica-Forming Proteins as Versatile Tools for One-Step Enzyme Immobilization on Siliceous Materials
- PMID: 39941072
- PMCID: PMC11818168
- DOI: 10.3390/ijms26031304
Exploiting Silica-Binding and Silica-Forming Proteins as Versatile Tools for One-Step Enzyme Immobilization on Siliceous Materials
Abstract
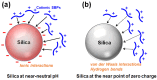
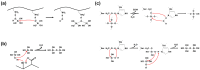
Enzyme immobilization has emerged as an essential technique in industrial applications of enzymes. Silica (SiO2) serves as a prominent support material for enzyme immobilization. Recent advancements have led to the development of various silica-binding proteins (SBPs) and silica-forming proteins (SFPs) that are invaluable tools in immobilizing enzymes on siliceous materials in a fast and simple manner. SBPs facilitate the immobilization of enzymes with controlled orientation on silica surfaces, while SFPs enable the biomimetic synthesis and encapsulation of enzymes within silica particles. In this review, we explore recent advances in the use of SBPs and SFPs in enzyme applications. We provide a comprehensive overview of their mechanisms and sequence characteristics relevant to enzyme immobilization. Additionally, we summarize the recombinant production and immobilization procedures for enzymes with SBPs or SFPs. We then categorize the available SBPs and SFPs into naturally occurring and artificially engineered types, presenting recent examples that demonstrate their utilization in enzyme immobilization. Our review highlights the strengths and limitations of various SBPs and SFPs and sheds light on future directions for the development of tailor-made biocatalytic silica.
Keywords: biomimetic silica; enzyme immobilization; fusion protein; silica-binding peptide; silica-forming peptide.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


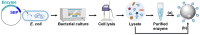

References
-
- Cavalcante F.T.T., Cavalcante A.L.G., de Sousa I.G., Neto F.S., dos Santos J.C.S. Current status and future perspectives of supports and protocols for enzyme immobilization. Catalysts. 2021;11:1222. doi: 10.3390/catal11101222. - DOI
-
- Hao X., Liu P., Chu X. Recent advances in the strategies of simultaneous enzyme immobilization accompanied by nanocarrier synthesis. Appl. Sci. 2024;14:3702. doi: 10.3390/app14093702. - DOI
-
- Costa J.R., Neto T., Pedrosa S.S., Sousa S.C., Azevedo-Silva J., Tavares-Valente D., Mendes A., Pintado M.E., Fernandes J.C., Oliveira A.L.S., et al. Biogenic silica microparticles as a new and sustainable cosmetic ingredient: Assessment of performance and quality parameters. Colloids Surf. B Biointerfaces. 2023;226:113305. doi: 10.1016/j.colsurfb.2023.113305. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

