Five-Year Comparative Study of Zygomatic and Subperiosteal Implants: Clinical Outcomes, Complications, and Treatment Strategies for Severe Maxillary Atrophy
- PMID: 39941332
- PMCID: PMC11818549
- DOI: 10.3390/jcm14030661
Five-Year Comparative Study of Zygomatic and Subperiosteal Implants: Clinical Outcomes, Complications, and Treatment Strategies for Severe Maxillary Atrophy
Abstract
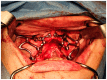
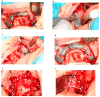
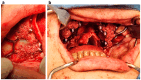
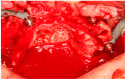
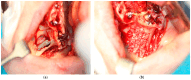
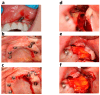
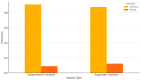
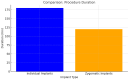
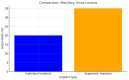
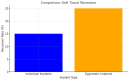
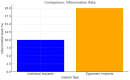
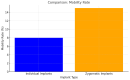
Background/Objectives: Severe maxillary atrophy presents challenges in maxillofacial rehabilitation. This study compares the clinical outcomes of zygomatic and subperiosteal implants, focusing on implant survival, soft tissue management, and postoperative complications over a five-year follow-up. Methods: A retrospective cohort study analyzed 150 patients divided into two groups based on the type of implant. Zygomatic implants were assessed for immediate functional loading, procedural efficiency, and complications such as sinus-related issues and orbital damage. Subperiosteal implants were evaluated for their customized design, keratinized mucosa integration, and adaptation to severe anatomical limitations. Statistical analyses, including Chi-square tests, were used to determine significant differences (p < 0.05). Results: This study demonstrated differences in complication rates (sinus-related complications: 12.4% for zygomatic implants; peri-implantitis: 5.6% for subperiosteal implants). Implant survival rates were comparable (zygomatic: 96.3%, subperiosteal: 97.1%, p = 0.278). Zygomatic implants demonstrated higher incidences of sinus-related complications (12.4%) and risks of orbital damage. Subperiosteal implants exhibited superior soft tissue stability with fewer cases of peri-implantitis (5.6%, p < 0.05). Procedural duration was shorter for zygomatic implants (177 min vs. 123 min); however, subperiosteal implants allowed for re-implantation after failure, providing flexibility that was unavailable with zygomatic implants. Conclusions: Zygomatic implants excel in immediate functional loading and reduced procedural time but require advanced surgical expertise to mitigate anatomical risks. Subperiosteal implants offer a safer, customizable solution, particularly in anatomically complex cases. These findings emphasize the importance of individualized treatment planning and technological advancements in implant design to optimize clinical outcomes for patients with severe maxillary atrophy.
Keywords: maxillary atrophy; subperiosteal implants; zygomatic implants.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Albrektsson T., Zarb G., Worthington P., Eriksson A.R. The long-term efficacy of currently used dental implants: A review. Int. J. Oral Maxillofac. Implant. 1986;1:11–25. - PubMed
-
- Aparicio C., Manresa C., Francisco K., Aparicio A., Nunes J., Claros P., Potau J.M. Zygomatic implants placed with two different techniques in edentulous maxillae: A 5-year retrospective study. Int. J. Oral Maxillofac. Implant. 2014;16:627–642.
-
- Chrcanovic B.R., Abreu M.H. Zygomatic implants for the rehabilitation of atrophic posterior maxilla: A review. Oral Maxillofac. Surg. 2013
LinkOut - more resources
Full Text Sources

