Development and Validation of a Quantitative Score for the Criteria Clinical Control in Stable COPD Proposed in the Spanish COPD Guidelines (GesEPOC): Results of the EPOCONSUL Audit
- PMID: 39941377
- PMCID: PMC11818294
- DOI: 10.3390/jcm14030707
Development and Validation of a Quantitative Score for the Criteria Clinical Control in Stable COPD Proposed in the Spanish COPD Guidelines (GesEPOC): Results of the EPOCONSUL Audit
Abstract
Introduction/Objective: the concept of clinical control of COPD is a measure proposed in the Spanish COPD Guidelines (GesEPOC), which aims to help clinicians assess the clinical status in order to adapt the treatment plan at follow-up. However, studies that have evaluated clinical practice reveal that the degree of control of COPD is not always assessed, which underlines the need to promote its assessment through a scoring system. To develop a scoring system that quantitatively assesses the validated criteria defining the degree of COPD control. Methods: this study used data from the EPOCONSUL audit in respiratory clinics across Spain. We included in this analysis all patients with a COPD clinical control grade estimated and reported by the physician at the visit, who had registered the criteria necessary to define the degree of clinical control validated and established in GesEPOC. Patients were randomly assigned to either the development or validation cohorts. The development cohort included 485 patients and the validation cohort included 341 patients. Score modelling was conducted using a multivariate logistic regression model, and calibration of the model and score was assessed using the Hosmer-Lemeshow goodness-of-fit test and GiViTi Calibration belts. The model and generated score's discrimination capacity were analyzed by calculating the Area Under the Curve (AUC). Results: the scoring system was developed using four criteria as predictors of poor clinical control of COPD reported by the treating physician:adjusted dyspnoea severity, use of rescue inhaler more than three times per week, walking less than 30 min per day, and COPD exacerbations in the last three months. The scoring system attributed scores from 0 to 8. Calibration was satisfactory in both development and validation cohorts, and the score's discrimination power, as indicated by the AUC, was 0.892. Conclusions: this scoring system provides an easy-to-use quantitative assessment of clinical control of COPD that we believe will help to measure COPD control and its evolution during patient follow-up. Future research will be needed to prospectively evaluate this score as a predictor of outcome.
Keywords: chronic obstructive pulmonary disease; degree clinical control; predicted probability; score.
Conflict of interest statement
MCR has received speaker fees from AstraZeneca, Bial, Chiesi, CSL Behring, GlaxoSmithKline, Menarini, Sanofi and Grifols, and consulting fees from GlaxoSmithKline, Sanofi, Grifols and Bial. JJSC has received speaker fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, FAES, GlaxoSmithKline, Menarini and Novartis, and consulting fees from Bi-al, Chiesi and GSK, and grants from GSK. MM has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Menarini, Kamada, Takeda, Zambon, CSL Behring, Specialty Therapeutics, Janssen, Grifols and Novartis, consulting fees from AstraZeneca, Atriva Therapeutics, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, CSL Behring, Inhibrx, Ferrer, Menarini, Mereo Biopharma, Spin Therapeutics, Specialty Therapeutics, ONO Pharma, Palobiofarma SL, Takeda, Novartis, Novo Nordisk, Sanofi, Zambon and Grifols and research grants from Grifols. BAN reports grants and personal fees from GSK, personal fees and non-financial support from Boehringer Ingelheim, personal fees and non-financial support from Chiesi, non-financial support from Laboratorios Menarini, grants, personal fees and non-financial support from AstraZeneca, personal fees from Gilead, personal fees and non-financial support from MSD, personal fees from Laboratorios BIAL, personal fees from Zambon, outside the submitted work; in addition, Dr. Alcázar-Navarrete has a patent P201730724 issued. JLLC has received honoraria for lecturing, scientific advice, participation in clinical studies or writing for publications for: AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, CSL Behring, Ferrer, Gebro, GlaxoSmithKline, Grifols, Menarini, Megalabs, Novartis and Rovi. MEFF no conflicts of interest. JLRH has received speaker fees from AstraZeneca, Bial, Boehringer Ingelheim, CSL Behring, GlaxoSmithKline, Zambon and Grifols, and consulting fees from Bial and Grifols. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
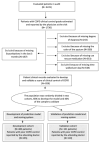


References
-
- Miravitlles M., Calle M., Molina J., Almagro P., Gómez J.-T., Trigueros J.A., Cosío B.G., Casanova C., López-Campos J.L., Riesco J.A., et al. Spanish COPD guidelines (GesEPOC) 2021: Updated pharmacological treatment of stable COPD. Arch. Bronconeumol. 2022;58:69–81. doi: 10.1016/j.arbres.2021.03.005. - DOI - PubMed
-
- Agustí A., Celli B.R., Criner G.J., Halpin D., Anzueto A., Barnes P., Bourbeau J., Han M.K., Martinez F.J., de Oca M.M., et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Arch. Bronconeumol. 2023;59:232–248. doi: 10.1016/j.arbres.2023.02.009. - DOI - PubMed
LinkOut - more resources
Full Text Sources

