Multikinase Treatment of Glioblastoma: Evaluating the Rationale for Regorafenib
- PMID: 39941744
- PMCID: PMC11816343
- DOI: 10.3390/cancers17030375
Multikinase Treatment of Glioblastoma: Evaluating the Rationale for Regorafenib
Abstract
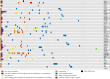
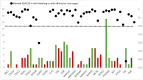
We explored the rationale for treating glioblastoma (GBM) with regorafenib. In 103 newly diagnosed GBM patients, we assessed mutations, copy number variants (CNVs), fusions, and overexpression in 46 genes encoding protein kinases (PKs) potentially targeted by regorafenib or its metabolites and performed a functional enrichment analysis to assess their implications in angiogenesis. We analyzed regorafenib's binding inhibitory activity and target affinity for these 46 PKs and focused on a subset of 18 genes inhibited by regorafenib at clinically achievable concentrations and on 19 genes involved in angiogenesis. Putative oncogenic alterations were defined as oncogenic/likely oncogenic mutations, oncogenic fusions, CNVs > 5, and/or gene overexpression. Regorafenib did not target all 46 PKs. For the 46-gene set, 40 genes (86.9%) and 73 patients (70.8%) harbored at least one alteration in genes encoding targetable PKs, but putative oncogenic alterations were present in only 34 patients (33%). In the 18-gene set, 18 genes (100%) and 48 patients (46.6%) harbored alterations, but putative oncogenic alterations were detected in only 26 patients (25.2%). Thirty patients (29.1%) had oncogenic alterations in the 18-gene set and/or in angiogenesis-related genes. Around 33% of patients had oncogenic alterations in any of the 46 potential targets. Additionally, the suboptimal dosing of regorafenib, due to its poor penetration of the blood-brain barrier, may reduce the likelihood of effectively targeting certain PKs. Future use of multi-target drugs must be guided by a thorough understanding of target presence, effective inhibition, and the drug's ability to reach brain tumors at adequate concentrations.
Keywords: glioblastoma; molecular target; multikinase treatment; regorafenib.
Conflict of interest statement
The authors declare no potential conflicts of interest for the data they are presenting. However, individual disclosures are as follows: Estela Pineda served in a consultant advisory role for Servier, Novocure, Global CNS Council, and GlaxoSmithKline and participated in the speakers’ bureau for Novocure. Marta Domenech was part of the speakers’ bureau for Bristol-Myers Squibb and Roche, received meeting assistance support from Lilly and Takeda, and has received a research project grant from Roche. Ainhoa Hernandez participated in the speakers’ bureau for Roche and received meeting assistance support from Roche, Sanofi, and Merck Sharp and Dohme. Carmen Balana served in an advisory role for MEDAC and received speakers’ bureau and meeting assistance support from Servier.
Figures

References
-
- Weller M., van den Bent M., Preusser M., Le Rhun E., Tonn J.C., Minniti G., Bendszus M., Balana C., Chinot O., Dirven L., et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2020;18:170–186. doi: 10.1038/s41571-020-00447-z. - DOI - PMC - PubMed
-
- Wen P.Y., Stein A., van den Bent M., De Greve J., Wick A., de Vos F., von Bubnoff N., van Linde M.E., Lai A., Prager G.W., et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutant low-grade and high-grade glioma (ROAR): A multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 2022;23:53–64. doi: 10.1016/S1470-2045(21)00578-7. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

