Tumor Necrosis Factor-Alpha Inhibitor Use and Malignancy Risk: A Systematic Review and Patient Level Meta-Analysis
- PMID: 39941759
- PMCID: PMC11815771
- DOI: 10.3390/cancers17030390
Tumor Necrosis Factor-Alpha Inhibitor Use and Malignancy Risk: A Systematic Review and Patient Level Meta-Analysis
Abstract
Over the last two decades, tumor necrosis factor-alpha inhibitors (TNF-Is) have become standard therapies for chronic inflammatory disorders, with an ongoing expansion of indications and off-label applications [...].
Keywords: TNF-alpha; adalimumab; cancer; certolizumab; etanercept; golimumab; infliximab; meta-analysis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
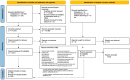







References
-
- Gerriets V., Goyal A., Khaddour K., Tumor Necrosis Factor Inhibitors Stat Pearls. [(accessed on 9 May 2022)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK482425/
-
- Urquhart L. Top Companies and Drugs by Sales in 2021. Nature Reviews Drug Discovery. [(accessed on 10 May 2022)]. Available online: https://www.nature.com/articles/d41573-022-00047-9. - PubMed
-
- Urquhart L. Top Companies and Drugs by Sales in 2017. Nature Reviews Drug Discovery. [(accessed on 10 May 2022)]. Available online: https://www.nature.com/articles/d41573-022-00047-9.
-
- Urquhart L. Top Companies and Drugs by Sales in 2020. Nature Reviews Drug Discovery. [(accessed on 10 May 2022)]. Available online: https://www.nature.com/articles/d41573-022-00047-9. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

