Evaluating Tumour Mutational Burden as a Key Biomarker in Personalized Cancer Immunotherapy: A Pan-Cancer Systematic Review
- PMID: 39941847
- PMCID: PMC11816366
- DOI: 10.3390/cancers17030480
Evaluating Tumour Mutational Burden as a Key Biomarker in Personalized Cancer Immunotherapy: A Pan-Cancer Systematic Review
Abstract
Background: Tumour mutational burden (TMB) is an emerging biomarker for predicting the efficacy of immune checkpoint inhibitors (ICIs) in cancer therapy. While its role is well established in lung cancer and melanoma, its predictive value for breast and prostate cancers remains unclear.
Objective: This systematic review aimed to assess the predictive value of TMB for ICI therapy across four major cancer types-lung, melanoma, breast, and prostate-and to explore factors contributing to the variability in its effectiveness as a biomarker.
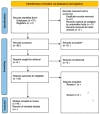
Methods: A systematic search and a review of the literature were conducted in accordance with PRISMA guidelines. Studies examining the relationship between TMB levels and clinical outcomes following ICI therapy in the specified cancers were analyzed. The data were synthesized to evaluate TMB's predictive value and identify gaps in the current research.
Results: High TMB consistently correlated with improved outcomes in lung cancer and melanoma, confirming its predictive utility in these cancers. Conversely, the findings for breast and prostate cancers were inconclusive. The variability in TMB's predictive value for these cancers suggests the need for complementary biomarkers or refined criteria to enhance its reliability. Methodological inconsistencies in TMB evaluation were also noted as a significant limitation.
Conclusions: TMB serves as a robust biomarker for predicting ICI response in lung cancer and melanoma, but demonstrates limited predictive utility in breast and prostate cancers. Future research should prioritize standardizing TMB assessment protocols and investigating additional biomarkers to improve treatment personalization for these cancer types.
Keywords: biomarkers; cancer immunotherapy; immune checkpoint inhibitors (ICIs); personalized medicine; systematic review; tumour mutational burden (TMB).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- FDA FDA Announces Approval, CMS Proposes Coverage of First Breakthrough-Designated Test to Detect Extensive Number of Cancer Biomarkers. [(accessed on 1 December 2024)];2017 Available online: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm587273.Htm.
-
- FDA FDA Unveils a Streamlined Path for the Authorization of Tumor Profiling Tests Alongside Its Latest Product Action. [(accessed on 1 December 2024)];2018 Available online: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm585347.Htm.
Publication types
LinkOut - more resources
Full Text Sources

