Patient-Derived Meningioma Organoids: A Reliable Model for Studying Human Tumor Pathophysiology
- PMID: 39941893
- PMCID: PMC11817449
- DOI: 10.3390/cancers17030526
Patient-Derived Meningioma Organoids: A Reliable Model for Studying Human Tumor Pathophysiology
Abstract
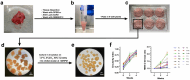
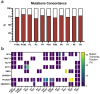
Introduction: Meningiomas are the most common primary central nervous system tumors, constituting 39.7% of intracranial tumors. Although generally benign, some exhibit aggressive behavior and risk of recurrence, necessitating adjuvant therapy and repeat surgical interventions. Molecular studies have identified tumor-driving mutations, leading to targeted therapies and clinical trials. However, translating preclinical findings into clinical success is often hindered by limitations in current meningioma tumor models. This study aims to develop and validate a standardized protocol for establishing patient-derived meningioma organoids (MEN-Os) that faithfully replicate human disease. Methods: MEN-Os were successfully established from 15 meningioma samples (11 grade 1, 4 grade 2) from neurosurgical resections using an optimized culture protocol. Histological and immunohistochemical analyses were used to assess the resemblance of MEN-Os to original tumor tissues. RNA sequencing compared transcriptional signatures between MEN-Os and corresponding patient-resected tissues. Results: MEN-Os were successfully established from patient-resected samples and maintained in culture for up to four weeks, showing stable growth and structural integrity. Histopathological analysis revealed that MEN-Os preserved key architectural features, including cellular organization, nuclear morphology, and proliferation rates. Immunohistochemical staining for meningioma-specific markers, such as the progesterone receptor, confirmed similar expression patterns to parental tumors. Transcriptomic profiling demonstrated that MEN-Os retained the transcriptional signatures of original tissues, including genes associated with meningioma pathology (NF2, CDKN2A, TP53). Differential expression and deconvolution analyses showed that MEN-Os contained diverse cell populations, including tumor and stromal cells, while preserving the immune microenvironment, as validated by histopathological and transcriptomic profiling. Conclusion: We established a robust, reproducible protocol for generating MEN-Os, which faithfully replicates the histopathological, molecular, and cellular characteristics of original tumors. MEN-Os provide a valuable model for studying meningioma biology and evaluating therapeutic strategies.
Keywords: meningioma; organoids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
-
- Chen W.C., Choudhury A., Youngblood M.W., Polley M.C., Lucas C.G., Mirchia K., Maas S.L.N., Suwala A.K., Won M., Bayley J.C., et al. Targeted gene expression profiling predicts meningioma outcomes and radiotherapy responses. Nat. Med. 2023;29:3067–3076. doi: 10.1038/s41591-023-02586-z. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

