Preparation and Biochemical Characterization of Penicillium crustosum Thom P22 Lipase Immobilization Using Adsorption, Encapsulation, and Adsorption-Encapsulation Approaches
- PMID: 39942541
- PMCID: PMC11821068
- DOI: 10.3390/molecules30030434
Preparation and Biochemical Characterization of Penicillium crustosum Thom P22 Lipase Immobilization Using Adsorption, Encapsulation, and Adsorption-Encapsulation Approaches
Abstract
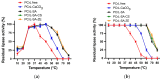
This work describes the immobilization and the characterization of purified Penicillium crustosum Thom P22 lipase (PCrL) using adsorption, encapsulation, and adsorption-encapsulation approaches. The maximum activity of the immobilized PCrL on CaCO3 microspheres and sodium alginate beads was shifted from 37 to 45 °C, compared with that of the free enzyme. When sodium alginate was coupled with zeolite or chitosan, the immobilization yield reached 100% and the immobilized PCrL showed improved stability over a wide temperature range, retaining all of its initial activity after a one-hour incubation at 60 °C. The immobilization of PCrL significantly improves its catalytic performance in organic solvents, its pH tolerance value, and its thermal stability. Interestingly, 95% and almost 50% of PCrL's initial activity was retained after 6 and 12 cycles, respectively. The characteristics of all PCrL forms were analyzed by X-ray diffraction and scanning electron microscopy combined with energy dispersive spectroscopy. The maximum conversion efficiency of oleic acid and methanol to methyl esters (biodiesel), by PCrL immobilized on CaCO3, was 65% after a 12 h incubation at 40 °C, while free PCrL generated only 30% conversion, under the same conditions.
Keywords: Penicillium crustosum lipase; esterification; hybrid carriers; immobilization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Pereira M.G., Velasco-Lozano S., Moreno-Perez S., Polizeli A.M., Heinen P.R., Facchini F.D.A., Vici A.C., Cereia M., Pessela B.C., Fernandez-Lorente G., et al. Different covalent immobilizations modulate lipase activities of Hypocrea pseudokoningii. Molecules. 2017;22:1448. doi: 10.3390/molecules22091448. - DOI - PMC - PubMed
-
- Chapman J., Ismail A.E., Dinu C.Z. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts. 2018;8:238. doi: 10.3390/catal8060238. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

