Design, Synthesis, and Anti-Tyrosinase, Anti-Melanogenic, and Antioxidant Activities of Novel (Z)-3-Benzyl-5-Benzylidene-2-Thioxothiazolidin-4-One Analogs
- PMID: 39942621
- PMCID: PMC11820736
- DOI: 10.3390/molecules30030517
Design, Synthesis, and Anti-Tyrosinase, Anti-Melanogenic, and Antioxidant Activities of Novel (Z)-3-Benzyl-5-Benzylidene-2-Thioxothiazolidin-4-One Analogs
Abstract
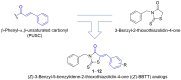
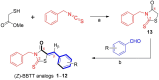
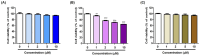
To discover novel anti-melanogenic compounds with tyrosinase inhibitory activity, (Z)-3-benzyl-5-benzylidene-2-thioxothiazolidin-4-one ((Z)-BBTT) analogs 1-12, designed based on the hybrid structure of a β-phenyl-α,β-unsaturated carbonyl motif and a 3-benzyl-2-thioxothiazolidin-4-one scaffold, were synthesized as novel tyrosinase inhibitors. Of the 12 analogs, 2 (6 and 8) showed mushroom tyrosinase inhibitory activity similar to that of kojic acid, a representative tyrosinase inhibitor, and 3 analogs (1-3) exhibited mushroom tyrosinase inhibitory activity that was more potent than that of kojic acid. In particular, analog 3 revealed highly potent inhibition with an IC50 value of 90 nM, which was 214 times lower than that of kojic acid (IC50 value = 19.22 μM). A kinetic study using mushroom tyrosinase and analogs 1-3 and 6 demonstrated that these analogs were competitive inhibitors, which was further supported by in silico studies. Analogs 1 and 3 have strong anti-melanogenic potency in B16F10 mammalian cells owing to their anti-tyrosinase activity without perceptible cytotoxicity in melanoma cells (B16F10) and the main epidermal cells (HaCaT). Moreover, analog 3 exhibited strong antioxidant capacity, scavenging reactive oxygen species, 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) cation radical, and 2,2-diphenyl-1-picrylhydrazyl radical, partially contributing to its anti-melanogenic effect. (Z)-BBTT analogs, including analog 3, may be promising candidates for inhibiting melanin production.
Keywords: (Z)-BBTT; B16F10 cells; melanin; tyrosinase; β-phenyl-α,β-unsaturated carbonyl.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Holick M.F. Sunlight, ultraviolet radiation, vitamin D and skin cancer: How much sunlight do we need? Adv. Exp. Med. Biol. 2014;810:1–16. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

