The Graphene Oxide/Gold Nanoparticles Hybrid Layers for Hydrogen Peroxide Sensing-Effect of the Nanoparticles Shape and Importance of the Graphene Oxide Defects for the Sensitivity
- PMID: 39942637
- PMCID: PMC11820113
- DOI: 10.3390/molecules30030533
The Graphene Oxide/Gold Nanoparticles Hybrid Layers for Hydrogen Peroxide Sensing-Effect of the Nanoparticles Shape and Importance of the Graphene Oxide Defects for the Sensitivity
Abstract
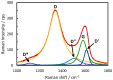
Graphene oxide (GO) and reduced graphene oxides (RGOs) show intrinsic electrocatalytic activity towards the electrocatalytic reduction of H2O2. Combining these materials with gold nanoparticles results in highly sensitive electrodes, with sensitivity in the nanomolar range because the electrocatalytic properties of GO and nanoparticles are synergistically enhanced. Understanding the factors influencing such synergy is crucial to designing novel catalytically active materials. In this contribution, we study gold nanostructures having shapes of nanospheres (AuNSs), nanourchins (AuNUs), and nanobowls (AuNBs) combined with GO or electrochemically reduced graphene oxide (ERGO). We investigate the amperometric responses of the hybrid layers to H2O2. The AuNUs show the highest sensitivity compared to AuNBs and AuNSs. All materials are characterized by electron microscopy and Raman spectroscopy. Raman spectra are deconvoluted by fitting them with five components in the 1000-1800 cm-1 range (D*, D, D", G, and D'). The interaction between nanoparticles and GO is visualized by the relative intensities of Raman bands (ID/IG) and other parameters in the Raman spectra, like various D", D* band positions and intensities. The ID/IG parameter is linearly correlated with the sensitivity (R2 = 0.97), suggesting that defects in the graphene structure are significant factors influencing the electrocatalytic H2O2 reduction.
Keywords: Raman intensities; gold nanoparticles; graphene; hydrogen peroxide sensing; non-enzymatic sensor; reactive oxygen species detection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Yu K., Li M., Chai H., Liu Q., Hai X., Tian M., Qu L., Xu T., Zhang G., Zhang X. MOF-818 Nanozyme-Based Colorimetric and Electrochemical Dual-Mode Smartphone Sensing Platform for in Situ Detection of H2O2 and H2S Released from Living Cells. Chem. Eng. J. 2023;451:138321. doi: 10.1016/j.cej.2022.138321. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

