Enantiopure Turbo Chirality Targets in Tri-Propeller Blades: Design, Asymmetric Synthesis, and Computational Analysis
- PMID: 39942707
- PMCID: PMC11819669
- DOI: 10.3390/molecules30030603
Enantiopure Turbo Chirality Targets in Tri-Propeller Blades: Design, Asymmetric Synthesis, and Computational Analysis
Abstract
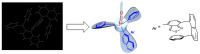
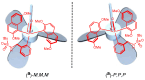
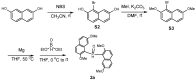
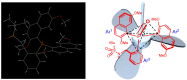
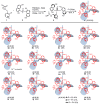
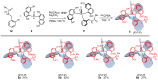
Enantiopure turbo chirality in small organic molecules, without other chiral elements, is a fascinating topic that has garnered significant interest within the chemical and materials science community. However, further research into and application of this concept have been severely limited by the lack of effective asymmetric tools. To date, only a few enantiomers of turbo chiral targets have been isolated, and these were obtained through physical separation using chiral HPLC, typically on milligram scales. In this work, we report the first asymmetric approach to enantiopure turbo chirality in the absence of other chiral elements such as central and axial chirality. This is demonstrated by assembling aromatic phosphine oxides, where three propeller-like groups are anchored to a P(O) center via three axes. Asymmetric induction was successfully carried out using a chiral sulfonimine auxiliary, with absolute configurations and conformations unambiguously determined by X-ray diffraction analysis. The resulting turbo frameworks exhibit three propellers arranged in either a clockwise (P,P,P) or counterclockwise (M,M,M) configuration. In these arrangements, the bulkier sides of the aromatic rings are oriented toward the oxygen atom of the P=O bond rather than in the opposite direction. Additionally, the orientational configuration is controlled by the sulfonimine auxiliary as well, showing that one of the Naph rings is pushed away from the auxiliary group (-CH2-NHSO2-tBu) of the phenyl ring. Computational studies were conducted on relative energies for the rotational barriers of a turbo target along the P=O axis and the transition pathway between two enantiomers, meeting our expectations. This work is expected to have a significant impact on the fields of chemistry, biomedicine, and materials science in the future.
Keywords: asymmetric synthesis; enantiopure turbo chirality; phosphine oxide; propeller blades; propeller chirality.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this article.
Figures
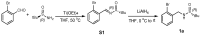
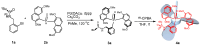
Similar articles
-
Amino Turbo Chirality and Its Asymmetric Control.Research (Wash D C). 2024 Sep 19;7:0474. doi: 10.34133/research.0474. eCollection 2024. Research (Wash D C). 2024. PMID: 39301263 Free PMC article.
-
Discovery of Staircase Chirality through the Design of Unnatural Amino Acid Derivatives.Research (Wash D C). 2024 Dec 19;7:0550. doi: 10.34133/research.0550. eCollection 2024. Research (Wash D C). 2024. PMID: 39703778 Free PMC article.
-
Atropisomers with Axial and Point Chirality: Synthesis and Applications.Acc Chem Res. 2022 Sep 20;55(18):2545-2561. doi: 10.1021/acs.accounts.2c00417. Epub 2022 Sep 9. Acc Chem Res. 2022. PMID: 36083117
-
Catalytic Asymmetric Synthesis of Atropisomers Bearing Multiple Chiral Elements: An Emerging Field.Angew Chem Int Ed Engl. 2024 Jan 15;63(3):e202311053. doi: 10.1002/anie.202311053. Epub 2023 Nov 21. Angew Chem Int Ed Engl. 2024. PMID: 37917574 Review.
-
Nanophotonic Platforms for Chiral Sensing and Separation.Acc Chem Res. 2020 Mar 17;53(3):588-598. doi: 10.1021/acs.accounts.9b00460. Epub 2020 Jan 8. Acc Chem Res. 2020. PMID: 31913015 Review.
References
-
- Eliel E.L., Wilen S.H. Stereochemistry of Organic Compounds. John Wiley & Sons; Hoboken, NJ, USA: 1994.
-
- Wagner I., Musso H. New Naturally Occurring Amino Acids. Angew. Chem. Int. Ed. Engl. 1983;22:816–828. doi: 10.1002/anie.198308161. - DOI
-
- Williams R.M. Synthesis of Optically Active [Alpha]-Amino Acids. Pergamon; Oxford, UK: 1989.
Grants and funding
LinkOut - more resources
Full Text Sources

