The Proteome Content of Blood Clots Observed Under Different Conditions: Successful Role in Predicting Clot Amyloid(ogenicity)
- PMID: 39942772
- PMCID: PMC11820299
- DOI: 10.3390/molecules30030668
The Proteome Content of Blood Clots Observed Under Different Conditions: Successful Role in Predicting Clot Amyloid(ogenicity)
Abstract
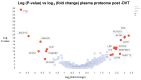
A recent analysis compared the proteome of (i) blood clots seen in two diseases-sepsis and long COVID-when blood was known to have clotted into an amyloid microclot form (as judged by staining with the fluorogenic amyloid stain thioflavin T) with (ii) that of those non-amyloid clots considered to have formed normally. Such fibrinaloid microclots are also relatively resistant to fibrinolysis. The proteins that the amyloid microclots contained differed markedly both from the soluble proteome of typical plasma and that of normal clots, and also between the diseases studied (an acute syndrome in the form of sepsis in an ITU and a chronic disease represented by Long COVID). Many proteins in the amyloid microclots were low in concentration in plasma and were effectively accumulated into the fibres, whereas many other abundant plasma proteins were excluded. The proteins found in the microclots associated with the diseases also tended to be themselves amyloidogenic. We here ask effectively the inverse question. This is: can the clot proteome tell us whether the clots associated with a particular disease contained proteins that are observed uniquely (or are highly over-represented) in known amyloid clots relative to normal clots, and thus were in fact amyloid in nature? The answer is in the affirmative in a variety of major coagulopathies, viz., venous thromboembolism, pulmonary embolism, deep vein thrombosis, various cardiac issues, and ischaemic stroke. Galectin-3-binding protein and thrombospondin-1 seem to be especially widely associated with amyloid-type clots, and the latter has indeed been shown to be incorporated into growing fibrin fibres. These may consequently provide useful biomarkers with a mechanistic basis.
Keywords: amyloid; clotting; cross-seeding; fibrils; fibrinaloid; proteomics.
Conflict of interest statement
E.P. is a named inventor on a patent application covering the use of fluorescence methods for microclot detection in Long COVID. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Pretorius E., Bester J., Vermeulen N., Alummoottil S., Soma P., Buys A.V., Kell D.B. Poorly controlled type 2 diabetes is accompanied by significant morphological and ultrastructural changes in both erythrocytes and in thrombin-generated fibrin: Implications for diagnostics. Cardiovasc. Diabetol. 2015;134:30. doi: 10.1186/s12933-015-0192-5. - DOI - PMC - PubMed
-
- Ząbczyk M., Natorska J., Janion-Sadowska A., Metzgier-Gumiela A., Polak M., Plens K., Janion M., Skonieczny G., Mizia-Stec K., Undas A. Prothrombotic fibrin clot properties associated with NETs formation characterize acute pulmonary embolism patients with higher mortality risk. Sci. Rep. 2020;10:11433. doi: 10.1038/s41598-020-68375-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

