Synthesis of ω-Methylsulfinyl- and ω-Methylsulfonylalkyl Glucosinolates
- PMID: 39942806
- PMCID: PMC11819801
- DOI: 10.3390/molecules30030704
Synthesis of ω-Methylsulfinyl- and ω-Methylsulfonylalkyl Glucosinolates
Abstract
General pathways were devised to synthesize ω-methylsulfinyl- and ω-methylsulfonylalkyl glucosinolates, which represent an important class of structurally homogeneous plant specialized metabolites. The first approach was based on the selective S-oxidation of ω-methylsulfanyl analogs previously obtained in our laboratory, producing the corresponding sulfoxide or sulfone counterparts in moderate yields. In an alternative approach, previously prepared ω-nitroalkyl methylsulfide precursors were selectively oxidized either to sulfoxides or to sulfones. The key-thiofunctionalized hydroximoyl chloride intermediates were prepared in situ from sulfoxides or sulfones using a nitronate chlorination strategy. A coupling reaction with 1-thio-β-d-glucopyranose was directly applied, followed by O-sulfation of the intermediate thiohydroximates. The final deprotection of the sugar moiety produced the target compounds, including renowned glucoraphanin and homologs, intended for further bioactivity investigations.
Keywords: glucosinolates; sulfones; sulfoxides; thiohydroximates.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

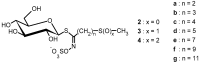





References
LinkOut - more resources
Full Text Sources

