A randomised, double-blind, placebo-controlled study to determine the analgesic efficacy, safety and tolerability of VPX638 administered topically to painful wounds
- PMID: 39943695
- PMCID: PMC11822243
- DOI: 10.1111/wrr.70008
A randomised, double-blind, placebo-controlled study to determine the analgesic efficacy, safety and tolerability of VPX638 administered topically to painful wounds
Abstract
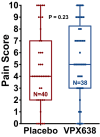
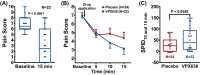
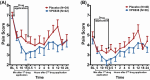
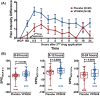
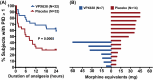
New analgesics are needed for painful wounds. Multiple reports suggest that topical sevoflurane may have analgesic effects. This placebo-controlled randomised trial evaluated the analgesic efficacy and safety of VPX638 (topical sevoflurane). Seventy-eight participants with painful wounds, were enrolled at eight Australian centres and randomly allocated to receive 2 × 5 mL of VPX638 (N = 39) or placebo (N = 40) during one wound dressing change. Numerical pain rating scores and use of opioids were recorded for 24 h. The primary endpoint was pain during wound cleaning, secondary endpoints evaluated pain for 24 h after drug application and opioids use. There was no significant difference in mean pain scores during wound cleaning between VPX638 and placebo (0.854; p = 0.23). The mean differences in summed pain intensity difference from baseline suggested VPX638 provided greater analgesia compared to placebo over 8 h (p < 0.02), 12 h (p < 0.01) and 24 h (p < 0.05) and significantly longer duration of analgesia, 24.3 h for VPX638 versus 7.1 h for placebo (p < 0.01). In the 24 h after drug administration, participants receiving VPX638 had a 50% decrease in opioid use over 24 h compared with placebo. VPX638 appeared safe and well-tolerated. In conclusion, this small placebo-controlled randomised trial suggested that VPX638 provides analgesia and is opioid-sparing for up to 24 h after wound cleaning. It supports the need for further evaluation of the benefit of VPX638 as a topical analgesic for painful wounds.
Keywords: analgesia; pain; sevoflurane; topical; wound.
© 2025 The Author(s). Wound Repair and Regeneration published by Wiley Periodicals LLC on behalf of The Wound Healing Society.
Conflict of interest statement
Vapogenix, Inc. staff received salary and stock options.
Figures
References
-
- Diligence M. Global wound prevalence forecast by type, 2016‐2026. Accessed July 20, 2018. https://blog.mediligence.com/2018/03/15/global-wound-prevalence-forecast...
-
- FDA . Classification of wound dressings combined with drugs. Accessed July 23, 2018. https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmateri...
-
- Phillips T, Stanton B, Provan A, Lew R. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol. 1994;31(1):49‐53. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

