Lineage-Specific Class-A GPCR Dynamics Reflect Diverse Chemosensory Adaptations in Lophotrochozoa
- PMID: 39943858
- PMCID: PMC11886862
- DOI: 10.1093/molbev/msaf042
Lineage-Specific Class-A GPCR Dynamics Reflect Diverse Chemosensory Adaptations in Lophotrochozoa
Abstract
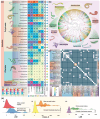
Sensing external chemosensory cues via Class-A G protein-coupled receptors (GPCRs) is crucial for a multitude of behavioral and biological functions, influencing animal evolution and ecological adaptations. While extensively studied in vertebrates and echinoderms, the role of GPCR-mediated chemoreception in major protostome clades like Lophotrochozoa remains obscure despite their remarkable ecological adaptations across diverse aquatic and terrestrial environments. Utilizing 238 lophotrochozoan genomes across eight phyla, we conducted a large-scale comparative genomics analysis to identify lineage-specific expansions of Class-A GPCR subsets that are likely adapted for chemoreception. Using phylogeny and orthology-inference-based clustering, we distinguished these expansions from conserved orthogroups of prospective endogenous ligand-binding Class-A GPCR subsets. Across phyla, lineage-specific expansions correlated with adaptations to various habitats, ecological niches, and lifestyles, while the influence of whole-genome duplications in driving these lineage-specific expansions appeared to be less significant. Species adapted to various coastal, freshwater, and terrestrial habitats across several classes of Mollusca, Annelida, and other analyzed phyla exhibit large and diverse lineage-specific expansions, while adaptations to extreme deep-sea environments, parasitic lifestyles, sessile behaviors, or alternative chemosensory mechanisms consistently exhibit reductions. Sequence heterogeneity, signatures of positive selection, and conformational flexibility in ligand-binding pockets further highlighted adaptations to environmental signals. In summary, the evolutionary dynamics of Class-A GPCRs in lophotrochozoans reveal a widespread pattern of lineage-specific expansions driven by adaptations for chemoreception across diverse environmental niches, mirroring the trends and prominent roles seen in deuterostome lineages. The comprehensive datasets spanning numerous genomes offer a valuable foundation for advancing GPCR-mediated chemoreception studies in Lophotrochozoa.
Keywords: Class-A GPCRs; Lophotrochozoa; chemoreception; environmental adaptations; genome evolution; lineage-specific expansions.
© The Author(s) 2025. Published by Oxford University Press on behalf of Society for Molecular Biology and Evolution.
Conflict of interest statement
Conflict of Interest: The authors declare that they have no competing interests.
Figures










References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

