The Protective Role of DDIT4 in Helicobacter pylori-induced Gastric Metaplasia Through Metabolic Regulation of Ferroptosis
- PMID: 39943905
- PMCID: PMC11937681
- DOI: 10.1016/j.jcmgh.2024.101448
The Protective Role of DDIT4 in Helicobacter pylori-induced Gastric Metaplasia Through Metabolic Regulation of Ferroptosis
Abstract
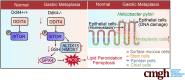
Background & aims: Helicobacter pylori (H pylori) infection is a significant factor leading to gastric atrophy, metaplasia and cancer development. Here, we investigated the role of the stress response gene DDIT4 in the pathogenesis of H pylori infection.
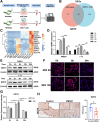
Methods: Cell lines, transgenic mice, and human tissue samples were implemented. Proteomics were performed on Ddit4+/+ and Ddit4-/- mice infected with H pylori strain PMSS1. C57BL/6 mice were administered with tamoxifen to induce gastric metaplasia. Stomach tissues were analyzed for histopathologic features, reactive oxygen species, Fe2+, lipid peroxidation, expression of DDIT4, and ferroptosis-related proteins.
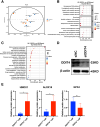
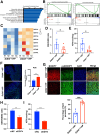
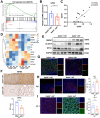
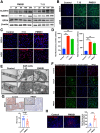
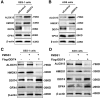
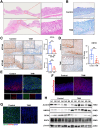
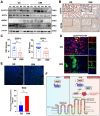
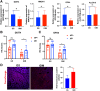
Results: DDIT4 expression was upregulated at 6 hours but significantly decreased at 24 hours in response to H pylori infection in gastric epithelial cells. Gastric DDIT4 were downregulated in INS-GAS mice at 4 months post H pylori infection. Notably, H pylori infection led to more severe gastric metaplasia lesion in Ddit4-knockout mice. The proteomic profiling revealed an increase in ferroptosis in the gastric tissues of infected Ddit4-deficient mice, compared with infected wild-type mice. Mechanistically, knockout of DDIT4 promoted H pylori-induced ferroptosis through the accumulation of lipid peroxides and ROS levels, and alterations in proteins such as GPX4, ALOX15, and HMOX1. Overexpression of DDIT4 counteracted H pylori-induced stem cell marker CD44V9 through modulation of ferroptosis. Similarly, in another mouse model of gastric metaplasia treated with tamoxifen, as well as in human GIM tissues, we observed the loss of DDIT4 and induction of ferroptosis.
Conclusions: Our results indicate that DDIT4 serves as a protective factor against H pylori-induced gastric metaplasia by metabolic resistance to ferroptosis.
Keywords: DDIT4; Ferroptosis; Gastric Metaplasia; H pylori; Metabolic Reprogramming.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Crowe S.E. Helicobacter pylori Infection. N Engl J Med. 2019;380:1158–1165. - PubMed
-
- Liabeuf D., Oshima M., Stange D.E., Sigal M. Stem cells, helicobacter pylori, and mutational landscape: utility of preclinical models to understand carcinogenesis and to direct management of gastric cancer. Gastroenterology. 2022;162:1067–1087. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

