Exclusive enteral nutrition for induction of remission in pediatric Crohn's disease: Short- and long-term tolerance and acceptance
- PMID: 39944105
- PMCID: PMC11810808
- DOI: 10.1002/jpr3.12163
Exclusive enteral nutrition for induction of remission in pediatric Crohn's disease: Short- and long-term tolerance and acceptance
Abstract
Objectives: In children with mild to moderate Crohn's disease (CD), exclusive enteral nutrition (EEN) is the first-line treatment. However, adherence to this therapeutic strategy remains challenging because of numerous psychosocial factors. This study aimed to evaluate the short-term acceptability and long-term tolerance of EEN.
Methods: A single-center retrospective study involving a pediatric population with CD was conducted at Robert-Debré Hospital in Paris between December 2023 and March 2024.
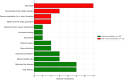
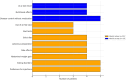
Results: Thirty-two patients responded to the questionnaire. developed specifically for this study. It included detailed sections on the EEN received, duration, observed consequences, difficulties encountered by patients and their families, and sociodemographic information. Twenty patients (62%) received oral treatment, while 12 (38%) required a nasogastric tube (NGT). Thirty-eight percent of the patients prematurely discontinued treatment. Most children reported difficulties related to taste, vomiting, and discomfort caused by the NGT. Fifty-nine percent of children would recommend treatment due to its rapid effectiveness, despite the challenges posed by the taste and exclusive nature of the diet. Thirty-two percent of patients reported persistent eating disorders (EDs) long after treatment discontinuation, and 12.5% reported social disorders. Despite strict treatment constraints, children managed to adapt and maintain their daily activities.
Conclusion: EEN has significant benefits for children with CD; however, its acceptability is mixed owing to dietary and social constraints. Adequate dietary and psychological support is crucial for improving adherence to treatment and preventing EDs in one third of our patients after treatment.
Keywords: children; inflammatory bowel disease; nutrition.
© 2024 The Author(s). JPGN Reports published by Wiley Periodicals LLC on behalf of The European Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Enteral nutritional therapy for induction of remission in Crohn's disease.Cochrane Database Syst Rev. 2018 Apr 1;4(4):CD000542. doi: 10.1002/14651858.CD000542.pub3. Cochrane Database Syst Rev. 2018. PMID: 29607496 Free PMC article.
-
Enteral nutritional therapy for induction of remission in Crohn's disease.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD000542. doi: 10.1002/14651858.CD000542.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2018 Apr 01;4:CD000542. doi: 10.1002/14651858.CD000542.pub3. PMID: 17253452 Updated.
-
Stem cell transplantation for induction of remission in medically refractory Crohn's disease.Cochrane Database Syst Rev. 2022 May 13;5(5):CD013070. doi: 10.1002/14651858.CD013070.pub2. Cochrane Database Syst Rev. 2022. PMID: 35556242 Free PMC article.
-
Enteral nutritional therapy for inducing remission of Crohn's disease.Cochrane Database Syst Rev. 2001;(3):CD000542. doi: 10.1002/14651858.CD000542. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2007 Jan 24;(1):CD000542. doi: 10.1002/14651858.CD000542.pub2. PMID: 11686966 Updated.
-
Efficacy of different dietary therapy strategies in active pediatric Crohn's disease: a systematic review and network meta-analysis.PeerJ. 2024 Dec 13;12:e18692. doi: 10.7717/peerj.18692. eCollection 2024. PeerJ. 2024. PMID: 39686992 Free PMC article.
References
-
- Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis. 2014;8(10):1179‐1207. - PubMed
LinkOut - more resources
Full Text Sources