Application of biomaterials in mesenchymal stem cell based endometrial reconstruction: current status and challenges
- PMID: 39944223
- PMCID: PMC11813782
- DOI: 10.3389/fbioe.2025.1518398
Application of biomaterials in mesenchymal stem cell based endometrial reconstruction: current status and challenges
Abstract
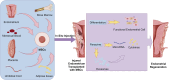
Severe endometrial injuries may cause thin endometrium and intrauterine adhesion in women which can result in uterine factor infertility. Current treatments, including surgical separation of adhesions and hormonal regeneration of the endometrium, often fail to prevent re-adhesion and achieve satisfactory reproductive results. Recently, mesenchymal stem cells (MSCs) have become a promising new treatment for IUA. However, challenges such as cell survival and transplantation limit the effectiveness of MSC therapy. Researchers have explored various approaches to enhance the therapeutic efficiency of MSCs. Among these, biomaterials have been frequently employed due to their biocompatibility, degradability, and ability to provide a conducive environment for cell growth. This review discusses the use of various biomaterials in MSC-based therapies for endometrial reconstruction and summarizes evidence from preclinical and clinical studies, highlighting the efficacy and safety of these biomaterials. The review also addresses future directions in this field, such as advances in biomaterial engineering, new biomaterials currently under investigation, and personalized medicine approaches. This review emphasizes the significance of biomaterials in MSC-based therapy for endometrial reconstruction and provides practical guidance for developing new materials and treatment protocols for clinical applications.
Keywords: biomaterials; endometrial reconstruction; intrauterine adhesion; mesenchymal stem cell based therapy; thin endometrium.
Copyright © 2025 He and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Evolution of biotechnological advances and regenerative therapies for endometrial disorders: a systematic review.Hum Reprod Update. 2024 Oct 1;30(5):584-613. doi: 10.1093/humupd/dmae013. Hum Reprod Update. 2024. PMID: 38796750 Free PMC article.
-
A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility.Acta Biomater. 2019 Jul 1;92:160-171. doi: 10.1016/j.actbio.2019.05.012. Epub 2019 May 7. Acta Biomater. 2019. PMID: 31075515
-
Minimally invasive delivery of human umbilical cord-derived mesenchymal stem cells by an injectable hydrogel via Diels-Alder click reaction for the treatment of intrauterine adhesions.Acta Biomater. 2024 Mar 15;177:77-90. doi: 10.1016/j.actbio.2024.02.001. Epub 2024 Feb 6. Acta Biomater. 2024. PMID: 38331133
-
Human embryonic stem cell-derived immunity-and-matrix-regulatory cells promote endometrial repair and fertility restoration in IUA rats.Stem Cell Res Ther. 2025 Apr 23;16(1):204. doi: 10.1186/s13287-025-04298-2. Stem Cell Res Ther. 2025. PMID: 40269948 Free PMC article.
-
Recent developments in bio-scaffold materials as delivery strategies for therapeutics for endometrium regeneration.Mater Today Bio. 2021 Feb 25;11:100101. doi: 10.1016/j.mtbio.2021.100101. eCollection 2021 Jun. Mater Today Bio. 2021. PMID: 34036261 Free PMC article. Review.
Cited by
-
Chitosan-based Biomaterials in Regenerative Medicine: Optimizing Mesenchymal Stem Cell Viability and Function.Stem Cell Rev Rep. 2025 Jul 24. doi: 10.1007/s12015-025-10901-z. Online ahead of print. Stem Cell Rev Rep. 2025. PMID: 40702223 Review.
References
-
- Alvarado-Gomez E., Martínez-Castañon G., Sanchez-Sanchez R., Ganem-Rondero A., Yacaman M. J., Martinez-Gutierrez F. (2018). Evaluation of anti-biofilm and cytotoxic effect of a gel formulation with Pluronic F-127 and silver nanoparticles as a potential treatment for skin wounds. Mater Sci. Eng. C Mater Biol. Appl. 92, 621–630. 10.1016/j.msec.2018.07.023 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources