Response to luspatercept can be predicted and improves overall survival in the real-life treatment of LR-MDS
- PMID: 39944234
- PMCID: PMC11814532
- DOI: 10.1002/hem3.70086
Response to luspatercept can be predicted and improves overall survival in the real-life treatment of LR-MDS
Abstract
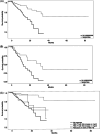
We explored the impact of luspatercept therapy on overall survival (OS) and possible predictors of response in low-risk (LR) myelodysplastic syndrome (MDS) patients. We evaluated 331 anemic patients treated with luspatercept. Hematological response (HI) was defined as (i) hemoglobin (Hb) increase of ≥1.5 g/dL in nontransfusion-dependent (NTD) patients, and (ii) red blood cell (RBC) transfusion independence (TI) with a concomitant Hb increase of ≥1.5 g/dL, or RBC-TI without an Hb increase of 1.5 g/dL, or >50% reduction in RBC transfusion burden (TB) for TD patients. Response was observed in 166 patients (50.2%), with significantly higher response in NTD and low TB versus high TB patients (p < 0.001). A significant correlation between lower Molecular International Prognostic Scoring System (IPSS-M) risk scores and response was observed. No statistically significant difference in HI was found in SF3B1-mutated versus wild-type MDS patients (53.8% vs. 40.1%, respectively). SF3B1mut hotspots (K700E vs. others) and variant allele frequencies (VAFs; <38% VAF vs. ≥38% VAF) did not impact on HI. SF3B1-mutated MDS with del5q showed inferior HI compared to other LR-MDS (p = 0.046). The median treatment duration overall was 35 weeks (20.86-90.29), the median time to response was 11 weeks (8.71-21.86), and the median duration of response was 65 weeks (26.5-114). After a median follow-up of 13 months, median OS was not reached (NR) for responders and 24 months for nonresponders (hazard ratio [HR] 0.25, 95% confidence interval 0.14-0.44, p < 0.001). This analysis of 331 luspatercept real-life-treated LR-MDS patients demonstrated a significant OS benefit upon luspatercept response. Low baseline RBC-TB and lower risk IPSS-M scores correlated with higher HI and could constitute predictive markers of response.
© 2025 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
The authors declare the following competing financial interests: V. S. has participated in advisory boards for Abbvie, Ascentage, BMS, Geron, Jazz, Keros, Novartis, Servier, and Syros, and has received travel grants from Abbvie and Jazz. R. K. has received consulting fees from Geron; has received research funding from Bristol Myers Squibb; has participated in advisory boards for AbbVie, Bristol Myers Squibb, CTI Biopharma, Jazz Pharmaceuticals, Novartis, PharmaEssentia, Rigel, Servio, and Taiho; and has received payment for speakers bureaus from AbbVie, CTI Biopharma, Jazz Pharmaceuticals, PharmaEssentia, and Servio. O. C. has worked for BMS and has received research funding from AbbVie and Jazz. M. G. D. P. has participated in advisory boards for BMS. E. P. has participated in advisory boards for BMS, GSK, Stemline, SoBi, Pharmaessentia, and Taiho and has received research funding from BMS and Incyte. D. A. S. has participated in consulting or advisory roles for Celyad, Agios, Abbvie, Aprea AB, Bristol‐Myers Squibb, Gilead Sciences, Intellia Therapeutics, Kite Pharma, Magenta Therapeutics, Novartis, and Syndax Speakers' Bureau for Agios, Incyte, and Bristol‐Myers Squibb; and has received research funding from Celgene and Jazz Pharmaceuticals. A. K. has participated as a consultant for MorphoSys, Abbvie, Karyopharm, Protagonist, and GSK and has received Honoraria from Incyte, Silence Therapeutics, Imago Biosciences, GSK, and BMS, as well as research support from Novartis, Geron, Janssen, GSK, BMS, Protagonist, and Blueprint.
Figures

References
-
- Oliva EN, Latagliata R, Danova M, et al. Darbepoetin for the treatment of anemia of myelodysplastic syndromes: efficacy and improvements in quality of life. Blood. 2006;108(11):2667. 10.1182/blood.v108.11.2667.2667 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
