LncRNA SNHG16 promotes LPS-induced human bronchial epithelial cell pyroptosis through miR-339-5p/NLRP1 axis mediation
- PMID: 39944259
- PMCID: PMC11811725
- DOI: 10.5114/ceji.2024.145876
LncRNA SNHG16 promotes LPS-induced human bronchial epithelial cell pyroptosis through miR-339-5p/NLRP1 axis mediation
Abstract
Introduction: Pyroptosis can aggravate lung injury in sepsis. It has been reported that lncRNA SNHG16 can regulate the inflammatory response. However, the role and underlying mechanism of SNHG16 in sepsis-induced pyroptosis and lung injury remain unclear.
Material and methods: To mimic septic lung injury in vitro, cells were treated with 1 µg/ml LPS. The Cell Counting Kit-8 (CCK-8) assay was performed to test cell viability. The lactate dehydrogenase (LDH) level was detected using a commercial kit. Interleukin (IL)-18 and IL-1 β secretion was tested using ELISA. Pyroptosis was investigated via flow cytometry. The relationship among SNHG16, miR-339-5p, and NLR family pyrin domain containing 1 (NLRP1) was explored using the dual luciferase assay.
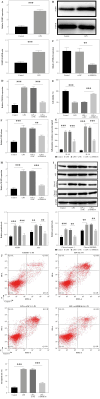
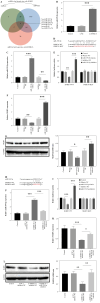
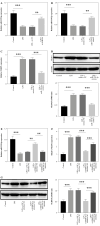
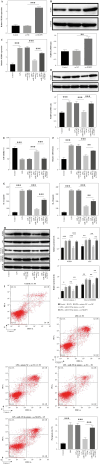
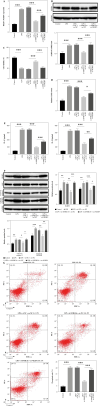
Results: LPS significantly upregulated the levels of SNHG16 and NLRP1 in BEAS-2B cells. In addition, LPS significantly induced pyroptosis in BEAS2B cells, while this phenomenon was reversed by SNHG16 silencing. SNHG16 could bind with miR-339-5p, and NLRP1 was found to be the downstream mRNA of miR-339-5p. SNHG16 silencing significantly abolished the LPS-induced upregulation of NLRP1 through miR-339-5p downregulation. The upregulation of miR-339-5p inhibited the pro-apoptotic effect of LPS on BEAS-2B cells, which was abolished by NLRP1 overexpression. Furthermore, the anti-pyroptotic effect of SNHG16 siRNA was abolished by NLRP1 upregulation.
Conclusions: SNHG16 silencing reversed LPS-induced pyroptosis in BEAS-2B cells via miR-339-5p/NLRP1 axis mediation. Our study might shed new light on exploring therapeutic strategies for the treatment of septic lung injury.
Keywords: NLRP1; SNHG16; miR-339-5p; pyroptosis.
Copyright © 2024 Termedia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
lncRNA NEAT1 mediates LPS-induced pyroptosis of BEAS-2B cells via targeting miR-26a-5p/ROCK1 axis.Kaohsiung J Med Sci. 2023 Jul;39(7):665-674. doi: 10.1002/kjm2.12681. Epub 2023 Apr 13. Kaohsiung J Med Sci. 2023. PMID: 37052185 Free PMC article.
-
miR-122-3p Alleviates LPS-Induced Pyroptosis of Macrophages via Targeting NLRP1.Ann Clin Lab Sci. 2023 Jul;53(4):578-586. Ann Clin Lab Sci. 2023. PMID: 37625833
-
Long noncoding RNA SNHG16 targets miR-146a-5p/CCL5 to regulate LPS-induced WI-38 cell apoptosis and inflammation in acute pneumonia.Life Sci. 2019 Jul 1;228:189-197. doi: 10.1016/j.lfs.2019.05.008. Epub 2019 May 7. Life Sci. 2019. PMID: 31071307 Review.
-
MiR-216a-5p alleviates LPS-induced inflammation in the human bronchial epithelial cell by inhibition of TGF-β1 signaling via down-regulating TGFBR2.Allergol Immunopathol (Madr). 2021 Sep 1;49(5):64-71. doi: 10.15586/aei.v49i5.458. eCollection 2021. Allergol Immunopathol (Madr). 2021. PMID: 34476924
-
Long non-coding RNA CDKN2B-AS1 enhances LPS-induced apoptotic and inflammatory damages in human lung epithelial cells via regulating the miR-140-5p/TGFBR2/Smad3 signal network.BMC Pulm Med. 2021 Jun 14;21(1):200. doi: 10.1186/s12890-021-01561-z. BMC Pulm Med. 2021. PMID: 34126975 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
