Valve Formation during Colostomy by Means of Spherical Implants Based on Titanium Nickelide Both Wrapping and Non-Wrapping the Serous-Muscular Layer of the Intestine
- PMID: 39944371
- PMCID: PMC11811834
- DOI: 10.17691/stm2023.15.6.06
Valve Formation during Colostomy by Means of Spherical Implants Based on Titanium Nickelide Both Wrapping and Non-Wrapping the Serous-Muscular Layer of the Intestine
Abstract
Imposition of a classic colostomy does not allow to control the passage of intestinal discharge. This shortcoming provides rationale for development of new materials for implants, as well as new techniques for valve formation. The aim of the study is to assess the possibility of using spherical implants based on titanium nickelide both wrapping and non-wrapping the serous-muscular layer of the intestine for valve formation during colostomy.
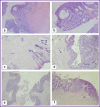
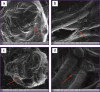
Materials and methods: Experiments were performed on 45 male Wistar rats with the average body weight of 587±10 g. Depending on the type of surgical intervention, all animals were divided into three groups. In the control group (n=15), a classic end colostomy was formed without spherical implants. In test group 1 (n=15), colostomy was formed using spherical implants made of titanium nickelide with wrapping the serous-muscular layer of the intestine; in test group 2 (n=15), the same procedure was performed without wrapping the serous-muscular layer. To assess the clinical condition of the animals, the authors monitored the body weight dynamics, food and water consumption, signs of discharge from the stoma, and recorded complications. The animals were euthanized on day 7, day 30, and day 60 of the experiment. During necropsy, the condition of the abdominal organs was assessed macroscopically with a special attention to the signs of adhesions. The severity of the inflammatory process in the area of surgical intervention was assessed histologically.
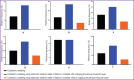
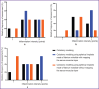
Results: The survival rate in three groups was 100%. In the group with the formation of a colostomy non-wrapping the serous-muscular layer, a good effect of regeneration in the stoma area was shown, the connection of the skin flap and the intestinal wall was complete. Macroscopically, adhesions and inflammatory processes of the peritoneum in the control and two test groups were minimal.
Conclusion: The present study shows the advantage of experimental modeling of colostomy using spherical titanium nickelide implants non-wrapping the serous-muscular layer of the colon compared to classical formation of colostomy. At that, wrapping the serous-muscular layer of the colon using spherical titanium nickelide implants is behind classical formation of a colostomy.
Keywords: colostomy; neosphincter; serous-muscular sphincter; titanium nickelide.
Conflict of interest statement
There are no conflicts of interest.
Figures



References
-
- Banashkevich Z., Yaven’ A., Shevchik M., Chezhnyakovska K. Surgical treatment of colostomy complications. Onkologiceskij zurnal 2010; 4(2):11–17.
-
- Halemani K., Shashidhara Y.N., D’Souza S.R.B. An evaluative study to assess the effectiveness of a video-assisted teaching module on knowledge and practice regarding homebased colostomy care of children among primary caregivers in selected hospital Lucknow, Uttar Pradesh. Indian J Surg Oncol 2021; 12(1):146–151, 10.1007/s13193-020-01268-3 - DOI - PMC - PubMed
-
- Kaprin A.D., Starinskiy V.V., Shakhzadova A.O. Zlokachestvennye novoobrazovaniya v Rossii v 2021 godu (zabolevaemost’ i smertnost’) [Malignant neoplasms in Russia in 2021 (morbidity and mortality)]. Moscow: MNIOI im. P.A. Gertsena — filial FGBU “NMITs radiologii” Minzdrava Rossii;. 2022; 252 p.
-
- Aliev S.A., Aliev E.S. Improving the methods of creating an end colostomy is a real way to prevent paracolostomy complications. Vestnik khirurgii im. I.I. Grekova 2015; 174(4):117–122. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
