Establishing rabbit critical-size bone defects to evaluate the bone-regeneration potential of porous calcium phosphate ceramics
- PMID: 39944476
- PMCID: PMC11813868
- DOI: 10.3389/fbioe.2024.1524133
Establishing rabbit critical-size bone defects to evaluate the bone-regeneration potential of porous calcium phosphate ceramics
Abstract
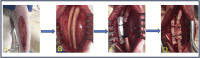
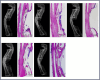
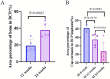
Critical-size bone defects (CSDs), which are those that do not self-repair in a given period, are essential for evaluating bone-regeneration strategies. We established CSDs models in the rabbit cranium and ulna, and the bone-regeneration capacities of porous calcium phosphate (CaP) ceramics were assessed. A 12.6-mm cranial defect was confirmed as a CSDs after 12 weeks, with submicron surface-structured biphasic calcium-phosphate (BCP) implants [consisting of 20% hydroxyapatite and 80% tricalcium phosphate (TCP)] demonstrating significantly higher bone formation (32.2% ± 10.6%) than micron surface-structured TCP (TCP-B) implants (17.8% ± 4.6%, p = 0.0121). Ulna defects (15.0 mm in length) failed to heal spontaneously within 24 weeks when the periosteum was removed from both the ulna and radius, and the radius was covered with an expanded polytetrafluoroethylene (ePTFE) membrane. No bone bridging (i.e., union) was observed in the BCP implants at 12 weeks, whereas 80% of BCP implants (four out of five) achieved union by 24 weeks. Furthermore, the bone area within the available space of BCP implants increased significantly from 19.3% ± 7.3% at 12 weeks to 37.7% ± 8.5% at 24 weeks (p = 0.0063), accompanied by significant BCP resorption (14.8% at 12 weeks and 30.2% at 24 weeks). This study offers two rabbit CSDs models for evaluating bone-regeneration strategies (including bone substitution), and the overall data obtained in the current study indicate the possibility of repairing CSDs with CaP ceramics demonstrating improved bone-forming ability given adequate implantation time.
Keywords: bone regeneration; bone substitutes; calcium phosphate ceramic; critical-size bone defect; submicron surface topography.
Copyright © 2025 Lei, Wu, Yuan, He, Wu, Chen, Liu, Zhang, de Bruijn, Xiang, Ji, Yuan and Li.
Conflict of interest statement
Authors JB and HY were employed by Kuros Biosciences BV. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Miscellaneous

