Clinical Practice Guidelines for Dementia: Recommendations for Cholinesterase Inhibitors and Memantine
- PMID: 39944527
- PMCID: PMC11813556
- DOI: 10.12779/dnd.2025.24.1.1
Clinical Practice Guidelines for Dementia: Recommendations for Cholinesterase Inhibitors and Memantine
Abstract
Background and purpose: This clinical practice guideline provides evidence-based recommendations for treatment of dementia, focusing on cholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists for Alzheimer's disease (AD) and other types of dementia.
Methods: Using the Population, Intervention, Comparison, Outcomes (PICO) framework, we developed key clinical questions and conducted systematic literature reviews. A multidisciplinary panel of experts, organized by the Korean Dementia Association, evaluated randomized controlled trials and observational studies. Recommendations were graded for evidence quality and strength using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology.
Results: Three main recommendations are presented: (1) For AD, cholinesterase inhibitors (donepezil, rivastigmine, galantamine) are strongly recommended for improving cognition and daily function based on moderate evidence; (2) Cholinesterase inhibitors are conditionally recommended for vascular dementia and Parkinson's disease dementia, with a strong recommendation for Lewy body dementia; (3) For moderate to severe AD, NMDA receptor antagonist (memantine) is strongly recommended, demonstrating significant cognitive and functional improvements. Both drug classes showed favorable safety profiles with manageable side effects.
Conclusions: This guideline offers standardized, evidence-based pharmacologic recommendations for dementia management, with specific guidance on cholinesterase inhibitors and NMDA receptor antagonists. It aims to support clinical decision-making and improve patient outcomes in dementia care. Further updates will address emerging treatments, including amyloid-targeting therapies, to reflect advances in dementia management.
Keywords: Alzheimer Disease; Cholinesterase Inhibitors; Dementia; Memantine; Practice Guidelines.
© 2025 Korean Dementia Association.
Conflict of interest statement
Conflict of Interest: The Steering and Working Committee members of the Clinical Practice Guideline Implementation Committee were checked for any consultancy or employment relationships with commercially related organizations, commercial equity holdings, research grants, honoraria, ownership of intellectual property rights related to the content of the guideline, or similar relationships involving their family or family-affiliated companies during the development or approval process of the guideline. All participating members confirmed that there were no conflicts of interest that could influence the development or approval process of this guideline.
Figures







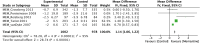
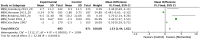

References
-
- Alzheimer A. Über eine eigenartige erkrankung der hirnrinde. All Z Psychiatr. 1907;64:146–148.
LinkOut - more resources
Full Text Sources

