Immune responses in rodent whole eye transplantation: elucidation and preliminary investigations into rejection diagnosis and monitoring
- PMID: 39944695
- PMCID: PMC11814173
- DOI: 10.3389/fimmu.2025.1475055
Immune responses in rodent whole eye transplantation: elucidation and preliminary investigations into rejection diagnosis and monitoring
Abstract
Background: Whole Eye Transplantation (WET) offers potential for vision restoration but is hindered by the complex challenge of immune rejection. Understanding and closely monitoring these immunological responses is crucial for advancing WET. This study delves into the timeline and nature of immune responses in a rodent model of WET without immunosuppression, aiming to elucidate a detailed picture of the immune landscape post-transplantation and establish innovative diagnostic and monitoring methods.
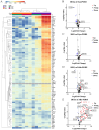
Methods: We employed a multi-faceted approach to analyze immune responses post-WET, including assessments of gross changes in corneal transparency, thickness, and skin condition. Histopathological examinations of both ocular and surrounding skin tissues provided insights into cellular changes, complemented by ocular RT-qPCR for molecular analysis. Serological analysis was employed to quantify cytokines, chemokines, and donor-specific antibodies, aiming to identify potential biomarkers correlating with WET rejection and to validate the presence of antibody-mediated rejection. These methodologies collectively contribute to the development of non-invasive diagnostic and monitoring strategies for WET.
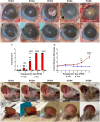
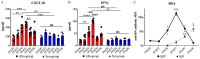
Results: Our study revealed a rapid and acute immune response following WET, characterized by an early innate immune response dominated by complement involvement, and infiltration of neutrophils and monocytes by post-operative day (POD) 2. This was succeeded by an acute T-cell-mediated immune reaction, predominantly involving T helper 1 (Th1) cells and cytotoxic T lymphocytes (CTLs). The presence of donor specific antibody (DSA) and indications of pyroptosis in the early phases of rejection were observed. Notably, the early elevation of serum CXCL10 by POD4, coupled with ocular CD3+ cell infiltration, emerged as a potential early biomarker for WET rejection. Additionally, corneal transparency grading proved effective as a non-invasive monitoring tool.
Conclusion: This study offers a first-time comprehensive exploration of immune responses in WET, unveiling rapid and complex rejection mechanisms. The identification of early biomarkers and the development of non-invasive monitoring techniques significantly advance our understanding of WET rejection. Additionally, these findings establish an essential baseline for future research in this evolving field.
Keywords: diagnostic strategies; immune rejection; monitoring techniques; non-invasive biomarkers; vascularized composite allotransplantation; whole eye transplantation.
Copyright © 2025 Li, Wang, Owens, Banaee, Chu, Jabbari, Lee, Khatter, Palestine, Su, Huang and Washington.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Blindness GBD. Vision Impairment C. Vision Loss Expert Group of the Global Burden of Disease S . Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden of Disease Study. Lancet Glob Health. (2021) 9:e130–e43. doi: 10.1016/S2214-109X(20)30425-3 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

