Age-Related Impairments in Immune Cell Efferocytosis and Autophagy Hinder Atherosclerosis Regression
- PMID: 39945065
- PMCID: PMC11936474
- DOI: 10.1161/ATVBAHA.124.321662
Age-Related Impairments in Immune Cell Efferocytosis and Autophagy Hinder Atherosclerosis Regression
Abstract
Background: Aging is a well-established risk factor for the development and progression of atherosclerosis, but the molecular mechanisms underlying this relationship remain poorly defined, and its role in atherosclerosis regression is unknown. To uncover age-related alterations that may impair atherosclerosis regression, we investigated the response of young and old macrophages to atherogenic lipoproteins in vitro and in vivo.
Methods: Metabolic and proteomic studies were performed in vitro using macrophages differentiated from the bone marrow of young or old mice. To test the role of immune cell aging in atherosclerosis regression, bone marrow from young and old donors was transplanted into irradiated young recipient mice expressing gain-of-function AAV-PCSK9 (adeno-associated virus-proprotein convertase subtilisin/kexin type 9). Following 14 weeks of Western diet feeding, atherosclerosis regression was induced by switching to a standard laboratory diet for 4 weeks.
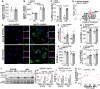
Results: Compared with young macrophages, old macrophages accumulated more lipid droplets upon lipid loading with the pro-atherogenic lipoprotein aggregated LDL (low-density lipoprotein), accompanied by a failure to proportionally induce autophagy and cholesterol efflux. Proteomic analysis of bone marrow-derived macrophages revealed that pathways related to endocytosis, engulfment, and phagocytosis were downregulated in old lipid-loaded macrophages. Functional studies confirmed a reduction in efferocytic capacity in old macrophages. In recipient mice transplanted with old bone marrow, atherosclerosis regression was impaired, as evidenced by inefficient resolution of circulating inflammatory cell levels, reduced activation of plaque autophagy and apoptotic cell clearance, and persistent plaque CD45+ and CD68+ content.
Conclusions: Aging impairs macrophage function through reduced efferocytosis and autophagy activation, limiting atherosclerosis regression. These results highlight the need to better define the mechanisms linking aging to atherosclerosis to develop targeted therapies for the aging population.
Keywords: atherosclerosis; cellular senescence; lipoproteins, LDL; macrophages; phagocytosis; plaque, atherosclerotic.
Conflict of interest statement
None.
Figures




References
-
- Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, Tokgözoğlu L, Lewis EF. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. doi: 10.1038/s41572-019-0106-z - PubMed
-
- Boucher DM, Vijithakumar V, Ouimet M. Lipid droplets as regulators of metabolism and immunity. Immunometabolism. 2021;3:e210020. doi: 10.20900/immunometab20210020 - PubMed
-
- Chistiakov DA, Melnichenko AA, Myasoedova VA, Grechko AV, Orekhov AN. Mechanisms of foam cell formation in atherosclerosis. J Mol Med (Berl). 2017;95:1153–1165. doi: 10.1007/s00109-017-1575-8 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

