Maintenance therapy with the FMS-like tyrosine kinase 3 inhibitor gilteritinib in patients with FMS-like tyrosine kinase 3-internal tandem duplication acute myeloid leukemia: A phase 2 study
- PMID: 39945223
- PMCID: PMC11822735
- DOI: 10.1002/cncr.35746
Maintenance therapy with the FMS-like tyrosine kinase 3 inhibitor gilteritinib in patients with FMS-like tyrosine kinase 3-internal tandem duplication acute myeloid leukemia: A phase 2 study
Abstract
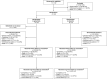
Background: The GOSSAMER phase 2 study assessed the FMS-like tyrosine kinase 3 (FLT3) inhibitor gilteritinib as maintenance therapy in patients with FLT3-internal tandem duplication (FLT3-ITD) acute myeloid leukemia (AML) in first complete remission without previous hematopoietic stem cell transplantation (HSCT).
Methods: Patients had to be within 2 months of their last consolidation cycle and have completed the recommended number of cycles per local practice. FLT3 inhibitors were allowed only during induction and/or consolidation. The primary end point was relapse-free survival (RFS). Secondary end points included overall survival (OS), event-free survival, and measurable residual disease (MRD).
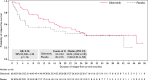
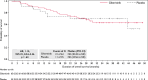
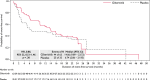
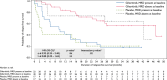
Results: In total, 98 patients were randomized (gilteritinib, n = 63; placebo, n = 35). RFS was not significantly different between the arms (hazard ratio, 0.74; 95% confidence interval, 0.41-1.34; p = .16). RFS rates for the gilteritinib and placebo arms were 68.5% and 55.3% at 1 year, 51.8% and 44.9% at 2 years, and 41.2% and 40.8% at 3 years, respectively. OS was not significantly different between the arms but may have been affected by subsequent AML therapies after discontinuation. In patients who received subsequent therapy (gilteritinib, 46.8%; placebo, 60.0%), a higher percentage of placebo-treated (57.1%) versus gilteritinib-treated patients (27.6%) underwent HSCT. At the end of treatment, 96.4% of gilteritinib-treated and 85.7% of placebo-treated patients had undetectable MRD. Relapsed placebo-treated (86.7%) versus gilteritinib-treated patients (34.8%) had a greater FLT3 mutational burden. No new significant safety concerns were noted.
Conclusions: The primary end point was not achieved; however, an observed trend toward potential benefit was noted in patients with FLT3-ITD AML who had not undergone prior HSCT.
Keywords: FLT3 inhibitor; FMS‐like tyrosine kinase 3–internal tandem duplication acute myeloid leukemia (FLT3‐ITD AML); gilteritinib; maintenance therapy; measurable residual disease.
© 2025 The Author(s). Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
Emmanuel Gyan has received honoraria from AbbVie, Astellas Pharma Inc., Bristol Myers Squibb, Janssen, Incyte, Roche, EUSA Pharma, Jazz Pharmaceuticals, Gilead, and Sanofi; research support from Novartis, Amgen, Bristol Myers Squibb, and Roche; travel support from AbbVie; and investigator fees from Pharmacyclics and Mundipharma. Mark D. Minden has received funding from the Canadian Institutes of Health Research for studies of the
Figures

Similar articles
-
Gilteritinib maintenance after allogeneic hematopoietic stem cell transplantation for relapsed/refractory acute myeloid leukemia with FLT3-internal tandem duplication mutation.Hematology. 2025 Dec;30(1):2509353. doi: 10.1080/16078454.2025.2509353. Epub 2025 Jun 17. Hematology. 2025. PMID: 40525981
-
Gilteritinib as Post-Transplant Maintenance for AML With Internal Tandem Duplication Mutation of FLT3.J Clin Oncol. 2024 May 20;42(15):1766-1775. doi: 10.1200/JCO.23.02474. Epub 2024 Mar 12. J Clin Oncol. 2024. PMID: 38471061 Free PMC article. Clinical Trial.
-
Myelomonocytic differentiation of leukemic blasts accompanied by differentiation syndrome in a case of FLT3-ITD-positive AML treated with gilteritinib.Hematology. 2021 Dec;26(1):256-260. doi: 10.1080/16078454.2021.1889111. Hematology. 2021. PMID: 33631087
-
Gilteritinib for the treatment of relapsed and/or refractory FLT3-mutated acute myeloid leukemia.Expert Rev Clin Pharmacol. 2019 Sep;12(9):841-849. doi: 10.1080/17512433.2019.1657009. Epub 2019 Aug 27. Expert Rev Clin Pharmacol. 2019. PMID: 31454267 Review.
-
The European Medicines Agency Review of Gilteritinib (Xospata) for the Treatment of Adult Patients with Relapsed or Refractory Acute Myeloid Leukemia with an FLT3 Mutation.Oncologist. 2020 Jul;25(7):e1070-e1076. doi: 10.1634/theoncologist.2019-0976. Epub 2020 Mar 10. Oncologist. 2020. PMID: 32154636 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

