Hypoxia-Induced Metabolic and Functional Changes in Oral CSCs: Implications for Stemness and Viability Modulation Through BNIP3-Driven Mitophagy
- PMID: 39945227
- PMCID: PMC11822456
- DOI: 10.1111/jcmm.70400
Hypoxia-Induced Metabolic and Functional Changes in Oral CSCs: Implications for Stemness and Viability Modulation Through BNIP3-Driven Mitophagy
Abstract
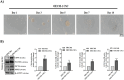
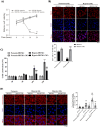
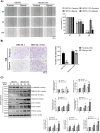
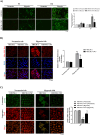
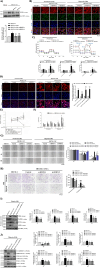
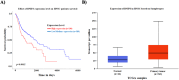
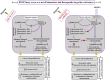
Oral squamous cell carcinomas (OSCCs), like several solid tumours, contain heterogeneous subpopulations of a small subset of cancer cells, termed cancer stem cells (CSCs), that are highly relevant to cancer metastasis and invasive properties. CSCs have also shown a high capacity to survive against various stressful environments, such as hypoxia. However, the molecular underpinnings behind the high potential of CSCs to survive under this stress remain unclear. The current study aimed to investigate the significance of autophagy systems in oral CSC maintenance and survival under stress conditions. Human OSCC cell lines OECM-1 and OECM-1 CSCs were cultured in different hypoxic time periods for proliferation and cytotoxicity analyses. The stemness property of CSCs is evaluated by sphere formation, transwell and wound healing assays protein expression of stemness, and epithelial-to-mesenchymal transition markers. Mitochondrial functions, including mitochondrial ROS generation, mitochondria dynamics, mitophagy, and mitochondrial metabolism (glycolysis and oxidative phosphorylation [OXPHOS]) were examined by western blotting, immunohistochemistry, and XF-seahorse assays, respectively. Under hypoxia, oral CSCs showed a higher proliferation rate with increased invasion/migration/EMT properties than OECM-1 cells. Further, hypoxia-induced BNIP3-driven mitophagy was activated in OECM-1 CSCs than in OECM-1 cells, which also triggered a metabolic shift towards OXPHOS, and BNIP3/-L silencing by siRNA significantly attenuated OECM-1 CSCs stemness features. TCGA data analyses also revealed a higher BNIP3 expression in head and neck squamous carcinoma patients' tumour samples associated with lower patient survival. Collectively, our results revealed a BNIP3/-L-driven autophagy contributes to the OECM-1 CSCs stemness features under hypoxia, suggesting a novel therapeutic strategy involving BNIP3 and autophagy inhibition in oral CSCs.
Keywords: BNIP3/‐L; Mitophagy; autophagy; cancer stem cells; oral squamous cell carcinoma; oxidative phosphorylation.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Mitophagy and cancer: role of BNIP3/BNIP3L as energetic drivers of stemness features, ATP production, proliferation, and cell migration.Aging (Albany NY). 2024 Jun 4;16(11):9334-9349. doi: 10.18632/aging.205939. Epub 2024 Jun 4. Aging (Albany NY). 2024. PMID: 38834039 Free PMC article.
-
Suppression of miR-204 enables oral squamous cell carcinomas to promote cancer stemness, EMT traits, and lymph node metastasis.Oncotarget. 2016 Apr 12;7(15):20180-92. doi: 10.18632/oncotarget.7745. Oncotarget. 2016. PMID: 26933999 Free PMC article.
-
Hypoxia-induced BNIP3 facilitates the progression and metastasis of uveal melanoma by driving metabolic reprogramming.Autophagy. 2025 Jan;21(1):191-209. doi: 10.1080/15548627.2024.2395142. Epub 2024 Sep 12. Autophagy. 2025. PMID: 39265983
-
Targeting redox regulation and autophagy systems in cancer stem cells.Clin Exp Med. 2023 Sep;23(5):1405-1423. doi: 10.1007/s10238-022-00955-5. Epub 2022 Dec 6. Clin Exp Med. 2023. PMID: 36473988 Review.
-
Epigenetic regulators governing cancer stem cells and epithelial-mesenchymal transition in oral squamous cell carcinoma.Curr Stem Cell Res Ther. 2015;10(2):140-52. doi: 10.2174/1574888x09666141020163700. Curr Stem Cell Res Ther. 2015. PMID: 25330402 Review.
References
-
- Singhavi H. R., Chakrabarti S., Singh A., et al., “Comparison of the Seventh and Eighth Editions American Joint Committee Cancer Classification System in Oral Cavity Squamous Cell Cancers,” International Journal of Cancer 146, no. 12 (2020): 3379–3384. - PubMed
-
- Warnakulasuriya S., “Global Epidemiology of Oral and Oropharyngeal Cancer,” Oral Oncology 45, no. 4–5 (2009): 309–316. - PubMed
-
- Ernani V. and Saba N. F., “Oral Cavity Cancer: Risk Factors, Pathology, and Management,” Oncology 89, no. 4 (2015): 187–195. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

