The pancreatic tumor microenvironment of treatment-naïve patients causes a functional shift in γδ T cells, impairing their anti-tumoral defense
- PMID: 39945298
- PMCID: PMC11834455
- DOI: 10.1080/2162402X.2025.2466301
The pancreatic tumor microenvironment of treatment-naïve patients causes a functional shift in γδ T cells, impairing their anti-tumoral defense
Abstract
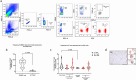
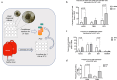
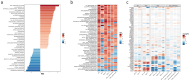
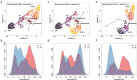
Pancreatic ductal adenocarcinoma (PDAC) presents a unique challenge for researchers due to its late diagnosis caused by vague symptoms and lack of early detection markers. Additionally, PDAC is characterized by an immunosuppressive microenvironment (TME), making it a difficult tumor to treat. While γδ T cells have shown potential for anti-tumor activity, conflicting studies exist regarding their effectiveness in pancreatic cancer. This study aims to explore the hypothesis that the PDAC TME hinders the anti-tumor capabilities of γδ T cells through blockade of cytotoxic functions. For this reason, we chose to enroll PDAC treatment-naive patients to avoid the possibility of therapy modifying the TME. By flow cytometry, our research findings indicate that the presence of γδ T cells among CD45+ cells in tumor tissue is lower compared to CD66+ cells, but higher than in blood. Circulating Vδ1 T cells exhibit a terminal effector memory phenotype (TEMRA) more than Vδ2 T cells. Interestingly, Vδ1 and Vδ2 T cells appear to be more prevalent at different stages of tumor development. In our in vitro culture using conditioned medium derived from Patient-derived organoids ;(PDOs), we observed a shift in expression markers in γδ T cells of healthy individuals toward an activation and exhaustion phenotype, as confirmed by scRNA-seq analysis extracted from a public database. A deeper understanding of γδ T cells in PDAC could be valuable for developing novel therapies aimed at mitigating the impact of the pancreatic tumor microenvironment on this cell population.
Keywords: Fine-needle biopsies; PDAC; gamma delta T cells; immune checkpoints; immunotherapy; patient derived organoids.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Chong YP, Peter EP, Lee FJM, Chan CM, Chai S, Ling LPC, Tan EL, Ng SH, Masamune A, Ghafar SAA, et al. Conditioned media of pancreatic cancer cells and pancreatic stellate cells induce myeloid-derived suppressor cells differentiation and lymphocytes suppression. Sci Rep. 2022;12(1):12315. doi:10.1038/s41598-022-16671-9. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
