A Half-Sandwich Os(II) Glucoconjugated NHC Complex as a Modulator of Amyloid Aggregation
- PMID: 39945504
- PMCID: PMC11863378
- DOI: 10.1021/acs.inorgchem.4c04823
A Half-Sandwich Os(II) Glucoconjugated NHC Complex as a Modulator of Amyloid Aggregation
Abstract
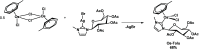
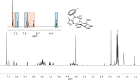
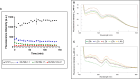
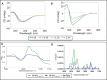
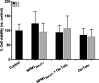
Herein, the effects of a novel half-sandwich Os(II) complex on the aggregation of an amyloid model system, derived from the C-terminal domain of the nucleophosmin 1 protein (NPM1264-277), were investigated. The thioflavin T (ThT) binding assay revealed that the complex [(η6-toluene)Os(NHCglu)Cl2] (where NHCglu is the N-heterocyclic carbene ligand 1-methyl-3-{2,3,4,6-tetra-O-acetyl-1-glucosyl}imidazol-2-ylidene), hence named Os-Tolu, was able to repress amyloid aggregation in a dose-dependent way. Conformational studies through circular dichroism (CD) and Fourier transform infrared (FTIR) spectroscopies clearly indicated that this inhibitory effect occurred through the stabilization of α-helical structures of monomeric NPM1264-277, thus hampering self-recognition. Electrospray ionization mass spectrometry (ESI-MS) studies evidenced, through the formation of coordination adducts, direct interactions of the amyloid peptide with the Os-glucoconjugate complex that, in turn, promote chemical modifications of the sequence further disfavoring the self-assembly process. Noticeably, the presence of Os-Tolu completely repressed the formation of amyloid fibers in scanning electron microscopy (SEM) analysis and induced a slight rescue of cell viability, in contrast to its reduction caused by the amyloid model in human SH-SY5Y neuroblastoma cells. These data strongly support the hypothesis of expanding the use of osmium-based agents to neurodegenerative diseases, positioning them as potential neurodrugs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
η6-Arene Ru(II) Complexes as Modulators of Amyloid Aggregation.Inorg Chem. 2024 Aug 26;63(34):16001-16010. doi: 10.1021/acs.inorgchem.4c02456. Epub 2024 Aug 11. Inorg Chem. 2024. PMID: 39129368
-
Heterobimetallic ferrocenyl-chromone compounds as selective inhibitors of amyloid aggregation.J Inorg Biochem. 2025 Sep;270:112932. doi: 10.1016/j.jinorgbio.2025.112932. Epub 2025 Apr 23. J Inorg Biochem. 2025. PMID: 40288002
-
A Comparative Study of the Effects of Platinum (II) Complexes on β-Amyloid Aggregation: Potential Neurodrug Applications.Int J Mol Sci. 2021 Mar 16;22(6):3015. doi: 10.3390/ijms22063015. Int J Mol Sci. 2021. PMID: 33809522 Free PMC article.
-
Metal-Complexes Bearing Releasable CO Differently Modulate Amyloid Aggregation.Inorg Chem. 2023 Jul 3;62(26):10470-10480. doi: 10.1021/acs.inorgchem.3c01522. Epub 2023 Jun 20. Inorg Chem. 2023. PMID: 37338927 Free PMC article.
-
Metal N-heterocyclic carbene complexes as potential antitumor metallodrugs.Chem Soc Rev. 2013 Jan 21;42(2):755-73. doi: 10.1039/c2cs35314h. Chem Soc Rev. 2013. PMID: 23147001 Review.
References
-
- Anthony E. J.; Bolitho E. M.; Bridgewater H. E.; Carter O. W. L.; Donnelly J. M.; Imberti C.; Lant E. C.; Lermyte F.; Needham R. J.; Palau M.; Sadler P. J.; Shi H.; Wang F.-X.; Zhang W.-Y.; Zhang Z. Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem. Sci. 2020, 11 (48), 12888–12917. 10.1039/D0SC04082G. - DOI - PMC - PubMed
-
- Annunziata A.; Cucciolito M. E.; Esposito R.; Ferraro G.; Monti D. M.; Merlino A.; Ruffo F. Five-coordinate Platinum(II) Compounds as Potential Anticancer Agents. Eur. J. Inorg. Chem. 2020, 918.10.1002/ejic.201900771. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

