A within-host infection model to explore tolerance and resistance
- PMID: 39945508
- PMCID: PMC12143882
- DOI: 10.7554/eLife.104052
A within-host infection model to explore tolerance and resistance
Abstract
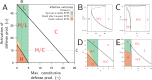
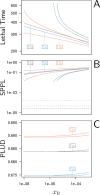
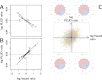
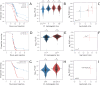
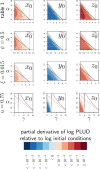
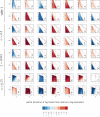
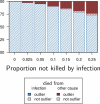
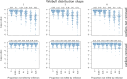
How are some individuals surviving infections while others die? The answer lies in how infected individuals invest into controlling pathogen proliferation and mitigating damage, two strategies respectively called resistance and disease tolerance. Pathogen within-host dynamics (WHD), influenced by resistance, and its connection to host survival, determined by tolerance, decide the infection outcome. To grasp these intricate effects of resistance and tolerance, we used a deterministic theoretical model where pathogens interact with the immune system of a host. The model describes the positive and negative regulation of the immune response, consider the way damage accumulate during the infection and predicts WHD. When chronic, infections stabilize at a Set-Point Pathogen Load (SPPL). Our model predicts that this situation can be transient, the SPPL being then a predictor of life span which depends on initial condition (e.g. inoculum). When stable, the SPPL is rather diagnostic of non-lethal chronic infections. In lethal infections, hosts die at a Pathogen Load Upon Death (PLUD) which is almost independent from the initial conditions. As the SPPL, the PLUD is affected by both resistance and tolerance but we demonstrate that it can be used in conjunction with mortality measurement to distinguish the effect of disease tolerance from that of resistance. We validate empirically this new approach, using Drosophila melanogaster and the pathogen Providencia rettgeri. We found that, as predicted by the model, hosts that were wounded or deficient of key antimicrobial peptides had a higher PLUD, while Catalase mutant hosts, likely to have a default in disease tolerance, had a lower PLUD.
Keywords: BLUD; D. melanogaster; bacteria; infection; infectious disease; microbiology; resistance; tolerance.
© 2025, Duneau, Lafont et al.
Conflict of interest statement
DD, PL, CL, NP, CF, XJ, NB, JF No competing interests declared
Figures














Update of
- doi: 10.1101/2021.10.19.464998
Similar articles
-
Genotype and diet shape resistance and tolerance across distinct phases of bacterial infection.BMC Evol Biol. 2014 Mar 22;14(1):56. doi: 10.1186/1471-2148-14-56. BMC Evol Biol. 2014. PMID: 24655914 Free PMC article.
-
A multi-faceted approach testing the effects of previous bacterial exposure on resistance and tolerance.J Anim Ecol. 2019 Apr;88(4):566-578. doi: 10.1111/1365-2656.12953. Epub 2019 Mar 6. J Anim Ecol. 2019. PMID: 30697699 Free PMC article.
-
Erratum: High-Throughput Identification of Resistance to Pseudomonas syringae pv. Tomato in Tomato using Seedling Flood Assay.J Vis Exp. 2023 Oct 18;(200). doi: 10.3791/6576. J Vis Exp. 2023. PMID: 37851522
-
The Fly Way of Antiviral Resistance and Disease Tolerance.Adv Immunol. 2018;140:59-93. doi: 10.1016/bs.ai.2018.08.002. Epub 2018 Sep 18. Adv Immunol. 2018. PMID: 30366519 Free PMC article. Review.
-
The damage threshold hypothesis and the immune strategies of insects.Infect Genet Evol. 2014 Jun;24:25-33. doi: 10.1016/j.meegid.2014.02.010. Epub 2014 Mar 12. Infect Genet Evol. 2014. PMID: 24614506 Review.
Cited by
-
Pathogen growth and virulence dynamics drive the host evolution against coinfections.Proc Natl Acad Sci U S A. 2025 Apr 29;122(17):e2412124122. doi: 10.1073/pnas.2412124122. Epub 2025 Apr 23. Proc Natl Acad Sci U S A. 2025. PMID: 40267133
References
-
- Araki M, Kurihara M, Kinoshita S, Awane R, Sato T, Ohkawa Y, Inoue YH. Anti-tumour effects of antimicrobial peptides, components of the innate immune system, against haematopoietic tumours in Drosophila mxc mutants. Disease Models & Mechanisms. 2019;12:dmm037721. doi: 10.1242/dmm.037721. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

