Rising incidence of radiation pneumonitis after adjuvant durvalumab in NSCLC patients treated with concurrent chemoradiotherapy
- PMID: 39945611
- PMCID: PMC11848943
- DOI: 10.2340/1651-226X.2025.42384
Rising incidence of radiation pneumonitis after adjuvant durvalumab in NSCLC patients treated with concurrent chemoradiotherapy
Abstract
Background and purpose: Adding adjuvant durvalumab to chemoradiotherapy (CRT) improves overall survival (OS) rates in locally advanced Non-Small-Cell Lung Cancer (NSCLC). However, recent data suggests that this new modality increases the incidence of radiation pneumonitis (RP). The aim of this study was to test the hypothesis that the incidence of RP after CRT and adjuvant durvalumab was higher than after CRT alone among patients with locally advanced NSCLC.
Materials and methods: The study population comprised all patients with NSCLC who completed CRT with curative intent between February 2013 and October 2020. From 2018 on, adjuvant durvalumab was administered in selected patients after completion of CRT. Patient and treatment data together with RP data (CTCAEv4.0, scored up to 9 months after CRT), were prospectively collected as part of our standard follow-up program.
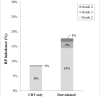
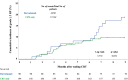
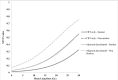
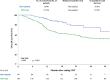
Results: A total of 284 patients were included, of which 90 (30.5%) received adjuvant durvalumab. Incidence of grade ≥2 RP increased in patients receiving durvalumab compared to CRT only (17.8% vs. 8.8%; p < 0.05), especially between 6 to 9 months after completing CRT. Adjuvant durvalumab and mean lung dose (MLD) were associated with a higher incidence of grade ≥2 RP (odds ratio [OR]: 2.43 and 1.14, respectively; p < 0.05). Current smoking was found to be a protective factor (OR: 0.38; p < 0.05).
Interpretation: Adjuvant durvalumab significantly increased the incidence of grade ≥2 RP in this real-world cohort of NSCLC patients. Patients receiving adjuvant durvalumab remain prone to develop grade ≥2 RP longer after completing CRT compared to patients treated with CRT only.
Conflict of interest statement
The department of Radiation Oncology of the UMCG has research collaborations with IBA, Philips, Mirada, RaySearch, Siemens, Elekta and Leoni.
T Jeroen N Hiltermann has collaborations with Roche, BMS, Astra Zeneca, MSD, Pfizer, GSK, Novartis, Merck Serono and Amgen.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

