Metastatic tumor growth in steatotic liver is promoted by HAS2-mediated fibrotic tumor microenvironment
- PMID: 39946200
- PMCID: PMC11957696
- DOI: 10.1172/JCI180802
Metastatic tumor growth in steatotic liver is promoted by HAS2-mediated fibrotic tumor microenvironment
Abstract
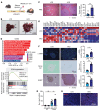
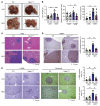
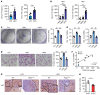
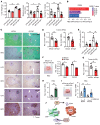
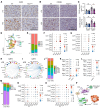
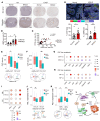
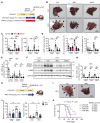
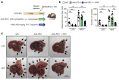
Steatotic liver enhances liver metastasis of colorectal cancer (CRC), but this process is not fully understood. Steatotic liver induced by a high-fat diet increases cancer-associated fibroblast (CAF) infiltration and collagen and hyaluronic acid (HA) production. We investigated the role of HA synthase 2 (HAS2) in the fibrotic tumor microenvironment in steatotic liver using Has2ΔHSC mice, in which Has2 is deleted from hepatic stellate cells. Has2ΔHSC mice had reduced steatotic liver-associated metastatic tumor growth of MC38 CRC cells, collagen and HA deposition, and CAF and M2 macrophage infiltration. We found that low-molecular weight HA activates Yes-associated protein (YAP) in cancer cells, which then releases connective tissue growth factor to further activate CAFs for HAS2 expression. Single-cell analyses revealed a link between CAF-derived HAS2 and M2 macrophages and CRC cells through CD44; these cells were associated with exhausted CD8+ T cells via programmed death-ligand 1 and programmed cell death protein 1 (PD-1). HA synthesis inhibitors reduced steatotic liver-associated metastasis of CRC, YAP expression, and CAF and M2 macrophage infiltration, and improved response to anti-PD-1 antibody. In conclusion, steatotic liver modulates a fibrotic tumor microenvironment to enhance metastatic cancer activity through a bidirectional regulation between CAFs and metastatic tumors, enhancing the metastatic potential of CRC in the liver.
Keywords: Extracellular matrix; Fibrosis; Hepatology; Liver cancer; Oncology.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

