Assessment of Anticancer and Antimicrobial Potential of Bioactive Metabolites and Optimization of Culture Conditions of Pseudomonas aurantiaca PB-St2 for High Yields
- PMID: 39947697
- PMCID: PMC11883349
- DOI: 10.4014/jmb.2311.11041
Assessment of Anticancer and Antimicrobial Potential of Bioactive Metabolites and Optimization of Culture Conditions of Pseudomonas aurantiaca PB-St2 for High Yields
Abstract
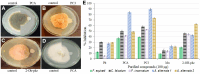
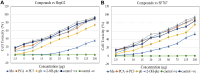
The following study aimed to characterize the biological potential of the purified compounds of Pseudomonas aurantiaca PB-St2. Optimization of temperature and incubation time of 32oC and 72 h yielded the highest crude extract weight and optical density of bacterial culture. HPLC analysis of the crude metabolite extract (purified using gravitational column chromatography) showed three fractions named as PC1, PC2, and PC3. HPLC-purified fractions were subjected to LC-MS/MS analysis and the data was compared using reference library. Fraction PC1 was identified as mupirocin, PC2 as phenazine-1-carboxylic acid (PCA), and PC3 as the mixture of three compounds including pyoluteorin, PCA and 2-hydroxyphenazine (2-OH-phz). Fungicidal potential of the purified compounds was assessed against phytopathogens including Fusarium equiseti, Fusarium incarnatum, Alternaria alternata, and Colletotrichum falcatum. Fraction PC3 showed the highest fungicidal activity of ~89%, whereas, the least antifungal activity (~27%) was noted for mupirocin. Antibacterial activity of the purified compounds against Gram-positive pathogen Bacillus cereus, and Gram-negative pathogens Pseudomonas aeruginosa, Salmonella enterica, and Klebsiella oxytoca was also assessed. Fraction PC3 demonstrated the highest antibacterial activity against B. cereus and P. aeruginosa showing 1.8 cm, and 0.9 cm zones of inhibition, respectively. Against K. oxytoca and S. enterica, the antibacterial activity of PB-St2 crude extract was slightly higher than the fraction PC3. The fraction PC3 also demonstrated the highest IC50 against HepG-2 and SF767 cancer cell lines at 25 μg and 20 μg concentrations, respectively. The multifaceted attributes of P. aurantiaca PB-St2 make it an ideal candidate for agricultural and pharmacological applications.
Keywords: Anticancer; P. aurantiaca; antimicrobial; bioactive metabolites; chromatography.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures






References
-
- Mishra J, Arora NK. Secondary metabolites of fluorescent pseudomonads in biocontrol of phytopathogens for sustainable agriculture. Appl. Soil Ecol. 2018;125:35–45. doi: 10.1016/j.apsoil.2017.12.004. - DOI
-
- Zboralski A, Biessy A, Savoie MC, Novinscak A, Filion M. Metabolic and genomic traits of phytobeneficial phenazineproducing Pseudomonas spp. are linked to rhizosphere colonization in Arabidopsis thaliana and Solanum tuberosum. Appl. Environ. Microbiol. 2020;86:e02443–19. doi: 10.1128/AEM.02443-19. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

