Applying positive end-expiratory pressure before and during endotracheal tube removal versus extubation with concomitant aspiration: protocol for the randomised controlled multicentre EXSUPEEP trial
- PMID: 39947817
- PMCID: PMC11831288
- DOI: 10.1136/bmjopen-2024-092354
Applying positive end-expiratory pressure before and during endotracheal tube removal versus extubation with concomitant aspiration: protocol for the randomised controlled multicentre EXSUPEEP trial
Abstract
Introduction: The optimal method for removing the endotracheal tube (ETT) during extubation in the intensive care unit (ICU) remains uncertain. Two methods are described for removing the ETT in ICU, namely the 'Traditional technique' with continuous aspiration during cuff deflation and ETT removal; and the 'PEEP' method, which consists in applying positive end-expiratory pressure (PEEP) before and during cuff deflation and ETT removal. Our hypothesis is that applying PEEP during extubation in the ICU would improve clinical outcome.
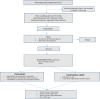
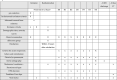
Methods and analysis: This is a prospective, multicentre, randomised, open-label, controlled, superiority trial, analysed by intention-to-treat, comparing ETT removal with concomitant suction vs application of PEEP before and during ETT removal. In total, 424 patients will be recruited and randomly assigned in a 1:1 ratio to one of two groups, according to the strategy of ETT removal. The primary outcome is the number of days free from any mechanical ventilation within 28 days following extubation. Secondary outcomes include the reintubation rate up to 7 days after ETT removal, the cumulative duration of non-invasive ventilation up to 7 days following extubation, the rate of acute respiratory failure, the rate of acquired pneumonia during the first 7 days following ETT removal, the length of stay in ICU and in hospital and all-cause mortality at 28 days following ETT removal.
Ethics and dissemination: The study was approved by the Ethics Committee 'CPP Ile de France II'. Patients will be included after providing written informed consent. The results will be submitted for publication in peer-reviewed journals, and in national and international congresses.
Trial registration number: NCT05147636.
Keywords: Adult intensive & critical care; Clinical Trial; Respiratory Therapy.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: The EXSUPEEP team and all investigators do not have any conflicts of interest to declare in connection with the study.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
