Facial Paralysis Algorithm: A Tool to Infer Facial Paralysis in Awake Mice
- PMID: 39947904
- PMCID: PMC11963837
- DOI: 10.1523/ENEURO.0384-24.2025
Facial Paralysis Algorithm: A Tool to Infer Facial Paralysis in Awake Mice
Abstract
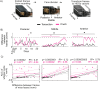
Facial paralysis is characterized by an injury to the facial nerve, causing the loss of the functions of the structures that it innervates, as well as changes in the motor cortex. Current models have some limitations for the study of facial paralysis, such as movement restriction, the absence of studying awake animals in behavioral contexts, and the lack of a model that fully evaluates facial movements. The development of an algorithm capable of automatically inferring facial paralysis and overcoming the existing limitations is proposed in this work. In C57/BL6J mice, we produced both irreversible and reversible facial paralysis. Video recordings were made of the faces of paralyzed mice to develop an algorithm for detecting facial paralysis applied to mice, which allows us to predict the presence of reversible and irreversible facial paralysis automatically. At the same time, the algorithm was used to track facial movement during gustatory stimulation and extracellular electrophysiological recordings in the anterolateral motor cortex (ALM). In the basal state, mice can make facial expressions, whereas the algorithm can detect this movement. Simultaneously, such movement is correlated with the activation in the ALM. In the presence of facial paralysis, the algorithm cannot detect movement. Furthermore, it predicts that the condition exists, and the neuronal activity in the cortex is affected with respect to the evolution of facial paralysis. This way, we conclude that the facial paralysis algorithm applied to mice allows for inferring the presence of experimental facial paralysis and its neuronal correlates for further studies.
Keywords: artificial vision; facial paralysis; nerve injury.
Copyright © 2025 Hernández et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






Similar articles
-
Automatic recognition of facial movement for paralyzed face.Biomed Mater Eng. 2014;24(6):2751-60. doi: 10.3233/BME-141093. Biomed Mater Eng. 2014. PMID: 25226980
-
Objective grading of facial paralysis using Local Binary Patterns in video processing.Annu Int Conf IEEE Eng Med Biol Soc. 2008;2008:4805-8. doi: 10.1109/IEMBS.2008.4650288. Annu Int Conf IEEE Eng Med Biol Soc. 2008. PMID: 19163791
-
Neurophysiology of ischemic facial nerve paralysis in an animal model.Acta Otolaryngol. 1991;111(5):934-42. doi: 10.3109/00016489109138433. Acta Otolaryngol. 1991. PMID: 1759581
-
The application of neuroprosthesis in restoration of unilateral facial nerve paralysis: A scoping review.J Plast Reconstr Aesthet Surg. 2025 Jan;100:67-81. doi: 10.1016/j.bjps.2024.11.006. Epub 2024 Nov 15. J Plast Reconstr Aesthet Surg. 2025. PMID: 39608139
-
Facial paralysis: a critical review of accepted explanation.Med Hypotheses. 2010 Mar;74(3):508-9. doi: 10.1016/j.mehy.2009.10.010. Epub 2009 Nov 11. Med Hypotheses. 2010. PMID: 19910126 Review.
Cited by
-
DeepFace: A High-Precision and Scalable Deep Learning Pipeline for Predicting Large-Scale Brain Activity from Facial Dynamics in Mice.bioRxiv [Preprint]. 2025 Jun 15:2025.06.10.658952. doi: 10.1101/2025.06.10.658952. bioRxiv. 2025. PMID: 40661434 Free PMC article. Preprint.
References
MeSH terms
LinkOut - more resources
Full Text Sources
