Detection of Overlooked Rare EGFR Mutations in Non-small Cell Lung Cancer Using Multigene Testing
- PMID: 39947926
- PMCID: PMC11825211
- DOI: 10.1111/1759-7714.70007
Detection of Overlooked Rare EGFR Mutations in Non-small Cell Lung Cancer Using Multigene Testing
Abstract
Background: Recognizing rare molecular variants of driver mutations poses a challenge in precision oncology, particularly for treatment of non-small cell lung cancer (NSCLC). In this study, we aimed to determine whether Oncomine Dx Target Test Multi-CDx System (ODxTT), the most widely used genetic test for NSCLC in Japan, potentially overlooks druggable EGFR mutations.
Materials and methods: Among 418 patients who underwent molecular testing using ODxTT at our hospital, 267 were diagnosed with adenocarcinoma. No mutations were reported in 82 of these cases. For these 82 cases, we searched for EGFR mutations in exons 18-21 by examining the binary alignment map file. Once a mutation was identified, its pathological significance was evaluated using the ClinVar database to determine whether ODxTT had overlooked any actionable EGFR mutations.
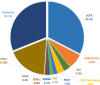
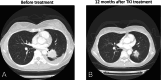
Results: Mutations in EGFR exons 19 and 18 were identified in six and four cases, respectively. Three, six, and none of these variants were detectable using the Cobas EGFR Mutation Test v2, Lung Cancer Compact Panel, and Amoy Dx, respectively. Of the 10 patients, five were subsequently treated with EGFR TKI; three showed partial response, one had stable disease, and one had progressive disease.
Conclusions: ODxTT failed to identify 10 actionable EGFR mutations, accounting for 12.2% (10/82) of the cases initially reported as not carrying actionable mutations. Therefore, comprehensive genomic profiling should be actively performed early in cases with high clinical suspicion of EGFR mutations.
Keywords: EGFR mutation; Oncomine Dx Target Test; next‐generation sequencing; non‐small cell lung cancer.
© 2025 The Author(s). Thoracic Cancer published by John Wiley & Sons Australia, Ltd.
Conflict of interest statement
NS has received honoraria for lectures at his institution from AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd., Novartis Pharma K.K., and Life Technologies Japan Ltd. TT reports fees paid to his institution for grants from Pfizer Japan Inc. and Takeda Pharmaceuticals, and personal fees for lectures from AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd., Roche Diagnostics, Takeda Pharmaceuticals, MSD K.K., and Novartis Pharma K.K. KS received personal fees for lectures from Life Technologies Japan Ltd., Chugai Pharmaceutical Co. Ltd., Yodosha Co. Ltd., Qiagen Inc., Takeda Pharmaceutical Co. Ltd., and Nippon Kayaku Co. Ltd. KT reports personal fees for lectures from AstraZeneca K.K., Eisai Co. Ltd., Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Merck Biopharma Co. Ltd., Bristol‐Myers Squibb Co. Ltd., MSD K.K., Takeda Pharmaceutical Co. Ltd., and Novartis Pharma K.K. KK reports fees paid to his institution for grants from Chugai Pharmaceutical Co. Ltd. and Takeda Pharmaceutical Co. Ltd. and personal fees for lectures from Chugai Pharmaceutical Co. Ltd., AstraZeneca K.K., and Daiichi Sankyo Co. Ltd. SS reports fees paid to his institution for grants from Nippon Boehringer Ingelheim Co. Ltd. and personal fees for lectures from Amgen Inc. and AstraZeneca K.K. JT reports personal fees for lectures from Boehringer Ingelheim Japan Inc., Bristol‐Myers Squibb Co. Ltd., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Eli Lilly Japan K.K., Janssen Pharmaceutical K.K., MSD K.K., Nihon Medi‐Physics Co. Ltd., Nippon Kayaku Co. Ltd., Taiho Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., AstraZeneca K.K., and Takeda Pharmaceuticals, and personal fees for participation on a Data Safety Monitoring Board or Advisory Board from AstraZeneca K.K. and AbbVie GK. KY reports fees paid to his institution for grants from Daiichi Sankyo Co. Ltd. and Boehringer Ingelheim Japan Inc., fees paid to his institution for royalties or licenses from Daiichi Sankyo Co. Ltd., personal fees for consultancy from Boehringer Ingelheim Japan Inc., personal fees for lectures from Amgen Inc., Chugai Pharmaceutical Co. Ltd., AstraZeneca K.K., Eli Lilly Japan K.K., Takeda Pharmaceutical Company Limited, and Merck Sharp & Dohme K.K., and patents pending with US20200115467 and US20200061031. KF reports fees paid to his institution for grants from Pfizer Japan Inc. and personal fees for lectures from Chugai Pharmaceutical Co. Ltd., KYORIN Pharmaceutical Co. Ltd., and IQVIA Inc. TM reports fees paid to his institution for grants from Boehringer Ingelheim Japan Inc. and Bridge Bio, personal fees for lectures from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol‐Myers Squibb Company, Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., Merck Sharp & Dohme K.K., Pfizer Japan Inc., Takeda Pharmaceuticals, Amgen Inc., Novartis Pharma K.K., Daiichi Sankyo Co. Ltd., and Thermo Fisher Scientific, and personal fees for participation on a data committee from Taiho Pharmaceutical Co. KN reports fees paid to his institution for grants from Nichirei Biosciences Corporation, NHO Osaka Minami Medical Center, West Japan Oncology Group, Nippon Boehringer Ingelheim Co., Hitachi Ltd., Otsuka Pharmaceutical Co., Thoracic Oncology Research Group, University Public Corporation Osaka, and Eli Lilly Japan K.K.; personal fees for consultancy from SymBio Pharmaceuticals K.K., Eli Lilly Japan K.K., and Otsuka Pharmaceutical Co. Ltd.; personal fees for lectures from Boehringer Ingelheim Japan, Yakult Honsha Co. Ltd., AstraZeneca K.K., Takeda Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Fujirebio Inc., Novartis Pharma K.K., Janssen Pharmaceutical K.K., Bristol‐Myers Squibb Co. Ltd., MSD K.K., Ono Pharmaceutical Co. Ltd., Daiichi Sankyo Inc., Pfizer Japan Inc., Invitae Japan K.K., Guardant Health, Nichirei Biosciences Inc., Amgen K.K., Maruho Co. Ltd., Merck Biopharma Co. Ltd., and Eli Lilly Japan K.K. HH reports fees paid to his institution for grants from IQVIA Services Japan K.K., Eisai Co. Ltd., Syneos Health Clinical K.K., EP‐CRSU Co. Ltd., EPS Corporation, Shionogi & Co. Ltd., Nippon Kayaku Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., GlaxoSmithKline K.K., MSD K.K., Sanofi K.K., Amgen Inc., Chugai Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., Bristol‐Myers Squibb Company, SRL Medisearch Inc., Janssen Pharmaceutical K.K., PRA Health Sciences Inc., CMIC Co. Ltd., Astellas Pharma Inc., Pfizer R&D Japan G.K., Ascent Development Services, Labcorp Development Japan K.K., Eisai Inc., Kobayashi Pharmaceutical Co. Ltd., Bayer Yakuhin Ltd., Pfizer Japan Inc., AstraZeneca K.K., AbbVie Inc., Daiichi Sankyo Co. Ltd., A2 Healthcare Corp., Novartis Pharma K.K., Eli Lilly Japan K.K., Merck Biopharma Co. Ltd., Medpace Japan K.K., Kyowa Kirin Co. Ltd., Japanese Gastric Cancer Association, Thoracic Oncology Research Group, Clinical Research Support Center Kyushu, West Japan Oncology Group, Japan Clinical Cancer Research Organization, Comprehensive Support Project for Oncological Research of Breast Cancer, EPS International Co. Ltd., Mebix Inc., Ono Pharmaceutical Co. Ltd., Mochida Pharmaceutical Co. Ltd., Covance Japan Inc., and Japan Clinical Research Operations Medical Research Support; personal fees for lectures from Ono Pharmaceutical Co. Ltd., Merck Biopharma Co. Ltd., Daiichi Sankyo Co. Ltd., 3H Clinical Trial Inc., AstraZeneca K.K., Novartis Pharma K.K., Chugai Pharmaceutical Co. Ltd., Bristol‐Myers Squibb Company, Eli Lilly Japan K.K., Amgen Inc., MSD K.K., Sysmex Corporation, Pfizer Japan Inc., Takeda Pharmaceutical Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., Janssen Pharmaceutical K.K., and Guardant Health Japan Corp.; personal fees for participation on a Data Safety Monitoring Board or Advisory Board from Bristol‐Myers Squibb Company, AbbVie Inc., Chugai Pharmaceutical Co. Ltd., Novocure K.K., AstraZeneca K.K., Daiichi Sankyo Co. Ltd., and Janssen Pharmaceutical K.K. during the conduct of the study. KN reports fees paid to his institution for grants from IQVIA Services Japan K.K., Syneos Health Clinical K.K., EPS Corporation, Nippon Kayaku Co. Ltd., EPS International Co. Ltd., Daiichi Sankyo Co. Ltd., Takeda Pharmaceutical Co. Ltd., MSD K.K., Ono Pharmaceutical Co. Ltd., Amgen Inc., Taiho Pharmaceutical Co. Ltd., EP‐CRSU Co. Ltd., Mebix Inc., Bristol‐Myers Squibb K.K., Janssen Pharmaceutical K.K., Pfizer R&D Japan G.K., Kobayashi Pharmaceutical Co. Ltd., Pfizer Japan Inc., Astellas Pharma Inc., Eisai Co. Ltd., AstraZeneca K.K., Mochida Pharmaceutical Co. Ltd., Labcorp Development Japan K.K. (Covance Japan Inc.), Japan Clinical Research Operations, Otsuka Pharmaceutical Co. Ltd., GlaxoSmithKline K.K., Sanofi K.K., Chugai Pharmaceutical Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., SRL Inc., Medical Research Support, Eli Lilly Japan K.K., Novartis Pharma K.K., CMIC Co. Ltd., Bayer Yakuhin Ltd., Shionogi & Co. Ltd., PRA Health Sciences Inc., and Ascent Development Services; personal fees for consultancy from Eli Lilly Japan K.K. and Ono Pharmaceutical Co. Ltd.; personal fees for lectures from Ono Pharmaceutical Co. Ltd., Amgen Inc., Nippon Kayaku Co. Ltd., AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., MSD K.K., Pfizer Japan Inc., Nippon Boehringer Ingelheim Co. Ltd., Taiho Pharmaceutical Co. Ltd., Bayer Yakuhin Ltd., Daiichi Sankyo Co. Ltd., Incyte Biosciences Japan, M3 Inc., Global Health Consulting Japan Co. Ltd., The Yomiuri Shimbun, Merck Biopharma Co. Ltd., TAIYO Pharma Co. Ltd., Takeda Pharmaceutical Co. Ltd., Life Technologies Japan Ltd., Neo Communication, Novartis Pharma K.K., Medical Mobile Communications Co. Ltd., Yodosha Co. Ltd., CMIC ShiftZero K.K., Japan Clinical Research Operations, CMIC Co. Ltd., Bristol‐Myers Squibb Company, Janssen Pharmaceutical K.K., Otsuka Pharmaceutical Factory Inc., and Hisamitsu Pharmaceutical Co. Ltd.; and patents planned, issued, or pending with Daiichi Sankyo Co. Ltd. The other authors declare no conflicts of interest.
Figures
References
-
- Reck M., Rodríguez‐Abreu D., Robinson A. G., et al., “Pembrolizumab Versus Chemotherapy for PD‐L1‐Positive Non‐Small‐Cell Lung Cancer,” New England Journal of Medicine 375, no. 19 (2016): 1823–1833. - PubMed
-
- Wu Y. L., Planchard D., Lu S., et al., “Pan‐Asian Adapted Clinical Practice Guidelines for the Management of Patients With Metastatic Non‐small‐Cell Lung cancer: A CSCO‐ESMO Initiative Endorsed by JSMO, KSMO, MOS, SSO and TOS,” Annals of Oncology 30, no. 2 (2019): 171–210. - PubMed
-
- Mitsudomi T., Morita S., Yatabe Y., et al., “Gefitinib Versus Cisplatin Plus Docetaxel in Patients With Non‐small‐Cell Lung cancer Harbouring Mutations of the Epidermal Growth Factor Receptor (WJTOG3405): An Open Label, Randomised Phase 3 Trial,” Lancet Oncology 11, no. 2 (2010): 121–128. - PubMed
-
- Mok T. S., Wu Y. L., Thongprasert S., et al., “Gefitinib or Carboplatin‐Paclitaxel in Pulmonary Adenocarcinoma,” New England Journal of Medicine 361, no. 10 (2009): 947–957. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

