Plyometric training increases thickness and volume of knee articular cartilage in mice
- PMID: 39948108
- PMCID: PMC11825961
- DOI: 10.1038/s41526-025-00458-z
Plyometric training increases thickness and volume of knee articular cartilage in mice
Abstract
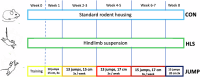
Degeneration and thinning of articular cartilage lead to osteoarthritis and may result from reduced joint loading during e.g. bed rest or as a result of microgravity during space flight. Anabolic physical exercises for cartilage are not well studied to date. We built an experimental apparatus for plyometric training with mice to test potential benefits of jumping for articular cartilage. The exercise group (JUMP) performed jump training for 9 weeks and was compared with sedentary mice (control, CON) and hindlimb-suspended (HLS) mice (to simulate reduced loading) for the same duration. Knee cartilage was assessed via 3-dimensional reconstruction of micro-CT scans and histology. We observed significant thinning and volume reduction of articular cartilage at the medial tibial-femoral point of contact in the HLS group. Clustering of chondrocytes was present in HLS. By contrast, the JUMP group showed both cartilage thickening and volume increase. We observed a similar trend on trabecular bone thickness and volume. Our results show that plyometric training can stimulate cartilage thickness and volume in mice. This suggests further investigation of this mode of exercise as a countermeasure to prevent cartilage atrophy in disuse scenarios such as long duration spaceflight, and for patients at risk of developing osteoarthritis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.S. is an Associate Editor for npj Microgravity. He had no role in the review process for this manuscript. All other authors declare no competing interests.
Figures





References
-
- Ramkumar, P. N. et al. The Effects of Space Microgravity on Hip and Knee Cartilage: A New Frontier in Orthopaedics. Surg. Tech. Inter.35, 421 (2019). - PubMed
-
- Liphardt, A.-M. et al. Vibration training intervention to maintain cartilage thickness and serum concentrations of cartilage oligometric matrix protein (COMP) during immobilization. Osteoarthr. Cartil.12, 1598 (2009). - PubMed
LinkOut - more resources
Full Text Sources

