Clinico-pathological factors predicting pathological response in early triple-negative breast cancer
- PMID: 39948122
- PMCID: PMC11825670
- DOI: 10.1038/s41523-025-00729-8
Clinico-pathological factors predicting pathological response in early triple-negative breast cancer
Abstract
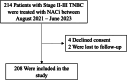
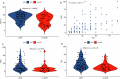
Pathological complete response (pCR) after neoadjuvant chemoimmunotherapy (NACi) is associated with improved patient outcomes in early triple-negative breast cancer (TNBC). This study aimed to identify factors associated with pCR after NACi. This cohort included all patients with stage II-III TNBC treated with NACi who underwent surgery at Institut Curie hospitals between 08/2021-06/2023. Among 208 patients, the overall pCR rate was 70% and was similar in ER < 1% (69%) and ER-low TNBC (73%, p = 0.6). In a multivariate model, Ki-67 ≥ 30% (OR 5.19 [1.73-17.3]), centralized TILs ≥ 30% (OR = 3.08 [1.42-7.04]), absence of DCIS at initial biopsy (OR = 2.56 [1.08-6.25]) and germline mutations in homologous recombination genes (OR = 9.50 [2.37-67.7]) remained strong independent predictors of pCR. These findings may guide treatment decisions in patients with TNBC undergoing NACi. Almost all patients with germline mutations in HR genes achieved pCR, supporting de-escalation trials. We suggest that ER-low tumors should be managed as TNBC tumors.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Dr. Helal, Dr. Djerroudi, Dr. Ramtohul, Dr. Seban, Dr. Carton, Pr. Bieche, Dr. Maxime Jin, Dr. Enora Laas, Dr. Claire Bonneau, and Dr. Luc Cabel do not declare a conflict of interest. Pr Anne Vincent-Salomon reported lectures honorarium: AstraZeneca, Daiichi Sankyo, Ibex, MSD, PRIMAA, Roche, Gilead; Advisory Board: AstraZeneca, Daiichi Sankyo, Ibex, PRIMAA, Roche; Research Funding: AstraZeneca, Ibex, MSD, MSD Avenir, Owkin; Stock option: Ibex. Dr Bello-Roufai reported Board from MSD, AstraZeneca, Lilly, Eisai, and travel fees: MSD, AstraZeneca, Eisai. Pr Bidard reported Research fundings: GE Healthcare, Pfizer, Prolynx, Menarini Silicon Biosystems, Merck KGaA, MSD, Novartis, Personalis, Pfizer, Roche, SAGA Diagnostics, and Tempus. Advisory boards for AstraZeneca, Daiichi-Sankyo, Exact Sciences, GE Healthcare, Gilead, Inatherys, Lilly, Menarini/Stemline, Novartis, Pfizer, Roche, SAGA Diagnostics; Speaker for AstraZeneca, Daiichi-Sankyo, Lilly, Menarini/Stemline, and Pfizer. Travel support from AstraZeneca, Daiichi-Sankyo, Pfizer, Novartis. Pr Cottu reported Honoraria: Pfizer, Roche, Lilly, Daiichi Sankyo, AstraZeneca, Gilead Sciences, Novartis, and NanoString Technologies. Consulting or Advisory Role: Pfizer, Lilly. Research funding: Pfizer. Travel, accommodations, and expenses: Roche, Pfizer, and Lilly. Dr Loirat reported Honoraria: AstraZeneca, Gilead Sciences Inc, Eli Lilly and Company, and MSD. Consulting or advisory fees: 4D Pharma, AstraZeneca, Gilead Sciences Inc., Immunomedics, Eli Lilly and Company, MSD Oncology, Novartis AG, Pfizer Inc., and Roche. Funding for travel, accommodations, and expenses: AstraZeneca, Gilead Sciences Inc., MSD, Pfizer Inc., and Roche. Dr. Lerebours reported Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events: AstraZeneca, Eisai, Gilead, Daiichi Sankyo, Lilly, Menarini, Novartis, Roche, and Seagen. Support for attending meetings and/or travel: Daiichi Sankyo, Gilead, Lilly, MSD, Novartis, Pfizer, and Seagen. Dr Kiavue reported a Travel Grant from Seagen. Dr. Romano reported Grants and other support from AstraZeneca, Replimune, Bristol Myers Squibb, and Fonds Amgen France pour la Science et l’Humain outside the submitted work.
Figures
References
-
- Foulkes, W. D., Smith, I. E. & Reis-Filho, J. S. Triple-negative breast cancer. N. Engl. J. Med.363, 1938–1948 (2010). - PubMed
-
- Hammond, M. E. H. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch. Pathol. Lab. Med.134, e48–e72 (2010). - PubMed
-
- Loibl, S. et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up☆. Ann. Oncol.35, 159–182 (2024). - PubMed
-
- Waks, A. G. & Winer, E. P. Breast cancer treatment: a review. JAMA321, 288–300 (2019). - PubMed
-
- Dent, R. et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res.13, 4429–4434 (2007). - PubMed
LinkOut - more resources
Full Text Sources

