Pancreatic endocrine and exocrine signaling and crosstalk in physiological and pathological status
- PMID: 39948335
- PMCID: PMC11825823
- DOI: 10.1038/s41392-024-02098-3
Pancreatic endocrine and exocrine signaling and crosstalk in physiological and pathological status
Abstract
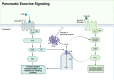
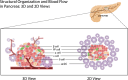
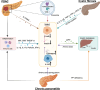
The pancreas, an organ with dual functions, regulates blood glucose levels through the endocrine system by secreting hormones such as insulin and glucagon. It also aids digestion through the exocrine system by secreting digestive enzymes. Complex interactions and signaling mechanisms between the endocrine and exocrine functions of the pancreas play a crucial role in maintaining metabolic homeostasis and overall health. Compelling evidence indicates direct and indirect crosstalk between the endocrine and exocrine parts, influencing the development of diseases affecting both. From a developmental perspective, the exocrine and endocrine parts share the same origin-the "tip-trunk" domain. In certain circumstances, pancreatic exocrine cells may transdifferentiate into endocrine-like cells, such as insulin-secreting cells. Additionally, several pancreatic diseases, including pancreatic cancer, pancreatitis, and diabetes, exhibit potential relevance to both endocrine and exocrine functions. Endocrine cells may communicate with exocrine cells directly through cytokines or indirectly by regulating the immune microenvironment. This crosstalk affects the onset and progression of these diseases. This review summarizes the history and milestones of findings related to the exocrine and endocrine pancreas, their embryonic development, phenotypic transformations, signaling roles in health and disease, the endocrine-exocrine crosstalk from the perspective of diseases, and potential therapeutic targets. Elucidating the regulatory mechanisms of pancreatic endocrine and exocrine signaling and provide novel insights for the understanding and treatment of diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

